| Date | November 2010 | Marks available | 4 | Reference code | 10N.3.sl.TZ0.B1 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | Deduce, Distinguish, and Draw | Question number | B1 | Adapted from | N/A |
Question
Foods such as rice, bread and potatoes are rich in carbohydrates. There are three main types of carbohydrate – monosaccharides, disaccharides and polysaccharides.
Glucose, \({{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}\), is a monosaccharide. When 0.85 g of glucose was completely combusted in a calorimeter, the temperature of 200.10 g of water increased from 20.20 °C to 27.55 °C. Calculate the energy value of glucose in \({\text{J}}\,{{\text{g}}^{ - 1}}\).
(i) Draw the straight chain structure of glucose.
(ii) Draw the structural formula of \(\alpha \)-glucose.
(iii) Distinguish between the structures of \(\alpha \)- and \(\beta \)-glucose.
(iv) Two \(\alpha \)-glucose molecules condense to form the disaccharide maltose. Deduce the structure of maltose.
One of the major functions of carbohydrates in the human body is as an energy source. State one other function of a carbohydrate.
Markscheme
\(\Delta T = 7.35\) (K/°C);
\(q( = mc\Delta T = {\text{200.10 g}} \times {\text{4.18 J}}\,{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}} \times {\text{7.35 K}}) = 6.15 \times {10^3}{\text{ J}}\) (per 0.85 g of glucose heated);
energy value \( = 7.2 \times {10^3}{\text{ (J}}\,{{\text{g}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
(i) 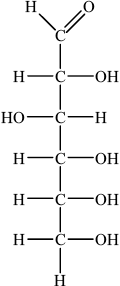
Accept CHO, CH2OH and OH groups on either side of the carbon chain provided OH on C3 is on the opposite side to OHs on C2, C4 and C5.
(ii) 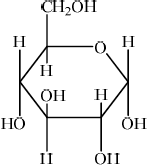 ;
;
Accept CH2OH and OH groups on either face, as long as OH on C3 is on the opposite face to the OH’s on C1, C2 and C4.
No mark awarded if HOCH2 is written, with H bonded to C or if HO is written for hydroxyl groups, with H bonded to C. Penalize this once only in (i), (ii) and (iv).
(iii) the OH on carbon-1/C-1 is inverted / difference in position of OH on carbon-1/C-1;
(iv) 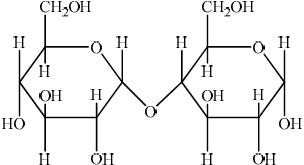 ;
;
energy reserve / can act as precursors in large number of metabolic reactions/for other biologically important molecules;
Examiners report
In (a) SD’s proved the major issue. Most candidates scored the mark for \(\Delta T\). The candidates who struggled made the following errors: incorrect mass (0.85 g) of water was used in \(q = mc\Delta T\), and failure to convert to J per g by dividing \(q\) by 0.85.
In (b)(i) many candidates struggled to draw the straight chain structure of glucose. In many instances the –OH groups were positioned incorrectly and some candidates were careless with bonding writing ‘-C–HO’ rather than ‘-C–OH’.
In (ii) a significant number of candidates mixed up the positions of the substituents on the two faces. In (iii) C1 was commonly omitted. It was surprising to see that quite a few candidates could not draw the structure of maltose in (iv). The most common mistake involved an incorrect linkage
Part (c) was answered well by the vast majority.

