| Date | November 2010 | Marks available | 3 | Reference code | 10N.3.hl.TZ0.A3 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Draw and State and explain | Question number | A3 | Adapted from | N/A |
Question
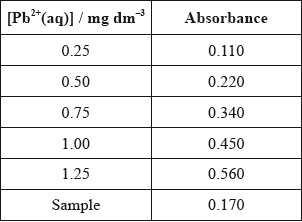
According to recommendations from the World Health Organization (WHO), the maximum allowed concentration of lead(II) cations, \({\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}}\), in drinking water is \({\text{0.001 mg}}\,{\text{d}}{{\text{m}}^{ - 3}}\). The tap water taken from a building was analysed using atomic absorption (AA) spectroscopy to determine the concentration of \({\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}}\). An AA spectrophotometer was calibrated and the following results were obtained.
Although both lead, Pb, and chromium, Cr, are metals, only chromium is classified as a transition metal and forms transition metal complexes, such as \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\).
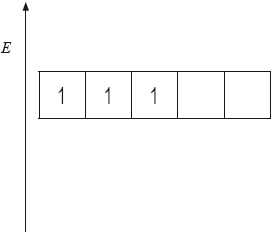
(i) The energy level diagram showing the electrons in the five 3d orbitals of a chromium atom is represented below. Draw the completed diagram showing the d orbitals in \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) after splitting.
(ii) State and explain what happens to the splitting of the d orbitals if the ligand is changed from \({{\text{H}}_{\text{2}}}{\text{O}}\) to \({\text{N}}{{\text{H}}_{\text{3}}}\).
Markscheme
(i) 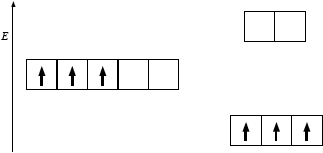
(ii) increases / greater;
Award [1] for one of the following:
NH3 has greater electron/charge density;
NH3 higher in spectrochemical series;
NH3 stronger base;
Allow converse argument for H2O.
Do not award M2 for stating that NH3 is a stronger ligand or has a smaller size.
If decreases is given for M1, then M2 cannot be scored.
Examiners report
(b) was very poorly answered and even the better candidates often scored zero marks here. In (i), it was very disappointing to see candidates failing to realise that in an octahedral crystal field the 3d sublevel splits into two sets of orbitals, a triply degenerate level which is lower in energy and a doubly degenerate level which is higher in energy. Many candidates put the triply degenerate level (the t2g level) at the same energy as the five-fold degenerate 3d sublevel, thereby clearly misunderstanding the splitting pattern. In addition, it was further very disappointing at HL to see candidates failing to apply Hund's rule of maximum multiplicity and placing two electrons in one orbital and one electron in another orbital. All three orbitals in the t2g level are degenerate and, hence, applying Hund‟s rule the electrons fill them singly first. Likewise in (ii), although some candidates stated that the splitting of the d-orbitals would increase if the ligand changed from water to ammonia, virtually no candidate mentioned a correct reason i.e. the fact that ammonia has greater charge density or is higher in the spectrochemical series. Clearly there were significant weaknesses in candidates‟ understanding of this topic overall.

