| Date | May 2011 | Marks available | 2 | Reference code | 11M.2.hl.TZ2.3 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Explain | Question number | 3 | Adapted from | N/A |
Question
The electron configuration of chromium can be expressed as \({\text{[Ar]4}}{{\text{s}}^{\text{x}}}{\text{3}}{{\text{d}}^{\text{y}}}\).
Hydrogen and nitrogen(II) oxide react according to the following equation.
\[2{{\text{H}}_2}{\text{(g)}} + {\text{2NO(g)}} \rightleftharpoons {{\text{N}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\]
At time = \(t\) seconds, the rate of the reaction is
\[{\text{rate}} = k{\text{[}}{{\text{H}}_2}{\text{(g)][NO(g)}}{{\text{]}}^2}\]
When concentrated hydrochloric acid is added to a solution containing hydrated copper(II) ions, the colour of the solution changes from light blue to green. The equation for the reaction is:
\[{{\text{[Cu(}}{{\text{H}}_2}{\text{O}}{{\text{)}}_6}{\text{]}}^{2 + }}{\text{(aq)}} + {\text{4C}}{{\text{l}}^ - }{\text{(aq)}} \to {{\text{[CuC}}{{\text{l}}_4}{\text{]}}^{2 - }}{\text{(aq)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}}\]
Explain what the square brackets around argon, [Ar], represent.
State the values of \(x\) and \(y\).
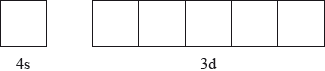
Annotate the diagram below showing the 4s and 3d orbitals for a chromium atom using an arrow, 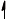 and
and 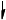 , to represent a spinning electron.
, to represent a spinning electron.
Explain precisely what the square brackets around nitrogen(II) oxide, [NO(g)], represent in this context.
Deduce the units for the rate constant \(k\).
Explain what the square brackets around the copper containing species represent.
Explain why the \({{\text{[Cu(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{2 + }}\) ion is coloured and why the \({{\text{[CuC}}{{\text{l}}_{\text{4}}}{\text{]}}^{2 - }}\) ion has a different colour.
Some words used in chemistry can have a specific meaning which is different to their meaning in everyday English.
State what the term spontaneous means when used in a chemistry context.
Markscheme
the electron configuration (of argon) / \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}\);
\(x = 1\) and \(y = 5\);
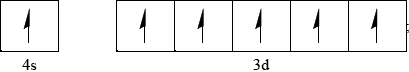
Accept all six arrows pointing down rather than up.
the concentration (of nitrogen(II) oxide);
Award [0] if reference made to equilibrium.
\({\text{mo}}{{\text{l}}^{ - 2}}{\text{d}}{{\text{m}}^{\text{6}}}{{\text{s}}^{ - 1}}/{\text{d}}{{\text{m}}^{\text{6}}}{\text{mo}}{{\text{l}}^{ - 2}}{{\text{s}}^{ - 1}}\);
Accept (mol–1 dm3)2s–1.
complex (ion) / the charge is delocalized over all that is contained in the brackets;
colour is due to energy being absorbed when electrons are promoted within the split d orbitals;
the colour observed is the complementary colour to the energy absorbed / OWTTE;
Accept either answer for first mark.
changing the ligand / coordination number / geometry changes the amount the d orbitals are split/energy difference between the d orbitals / OWTTE;
the reaction gives out (Gibbs Free) energy that can do work;
\(\Delta G\) for the reaction has a negative value;
a reaction that occurs without adding energy (beyond that required to overcome energy barrier);
Examiners report
Most candidates were familiar with the use of square brackets to represent noble gas electron configurations and concentrations in rate expressions and it was encouraging to see candidates give a correct orbital diagram with the d electrons unpaired.
A significant number of students were unaware of the exceptional nature of the electron configuration for chromium.
A significant number of students were unaware of the exceptional nature of the electron configuration for chromium, but were able to gain the mark in (a) (iii) with ecf.
The understanding of the use of square bracket to represent complex ions was limited.
Many candidates omitted the \({{\text{s}}^{ - 1}}\) in the units for the rate constant.
(c) (ii) proved to be more challenging with many candidates mixing up sub-shells with orbitals and absorption with emission spectra.
Many candidates were familiar with the use of the term spontaneous when used in a chemical context.

