| Date | November 2012 | Marks available | 3 | Reference code | 12N.3.hl.TZ0.A5 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Describe | Question number | A5 | Adapted from | N/A |
Question
The concentration of transition metal complexes in water can be determined by visible and ultraviolet (UV-Vis) spectroscopy.
Two octahedral chromium complexes are \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{2 + }}\) and \[{{\text{(Cr(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\). Describe how the increase in oxidation state from Cr(II) to Cr(III) and the change in ligand from water to ammonia will affect the splitting of the d orbitals and the frequency of the light these complexes absorb.
One of the following organic compounds is colourless while the other is orange.
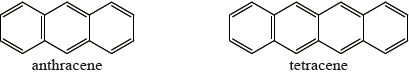
Predict, with reference to conjugation of double bonds, which compound (anthracene or tetracene) will absorb visible light and, therefore, be coloured.
Markscheme
increase in oxidation state causes greater splitting;
change from \({{\text{H}}_2}{\text{O}}\) to \({\text{N}}{{\text{H}}_3}\) causes greater splitting;
the greater the splitting, the higher the frequency (of absorbed light);
(complexes of) Cr(III) absorb higher-frequency light than (complexes of) Cr(II) /
(complexes with) \({\text{N}}{{\text{H}}_3}\) absorb higher-frequency light than (complexes with) \({{\text{H}}_2}{\text{O}}\);
Allow converse statements and OWTTE throughout.
tetracene and greater number of conjugated (double) bonds/larger delocalized system / OWTTE;
Examiners report
Option A proved to be very popular. Some candidates had difficulty explaining the purpose of the monochromator and some muddled Qualitative and Quantitative, but a reasonable proportion explained the latter. Many students were able to describe the practical method of column chromatography but were not able to explain the process in terms of adsorption, partition and retention. While many candidates knew about ‘d’ orbital splitting some forgot to explain the change in magnitude of the splitting, and a significant few thought that fewer ‘d’ electrons in the \({\text{C}}{{\text{r}}^{3 + }}\) ion would cause less repulsion and so less splitting.
Option A proved to be very popular. Some candidates had difficulty explaining the purpose of the monochromator and some muddled Qualitative and Quantitative, but a reasonable proportion explained the latter. Many students were able to describe the practical method of column chromatography but were not able to explain the process in terms of adsorption, partition and retention. While many candidates knew about ‘d’ orbital splitting some forgot to explain the change in magnitude of the splitting, and a significant few thought that fewer ‘d’ electrons in the \({\text{C}}{{\text{r}}^{3 + }}\) ion would cause less repulsion and so less splitting.

