| Date | May 2010 | Marks available | 3 | Reference code | 10M.2.hl.TZ2.6 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Explain | Question number | 6 | Adapted from | N/A |
Question
The periodic table shows the relationship between electron configuration and the properties of elements and is a valuable tool for making predictions in chemistry.
The ten elements in the first-row d-block have characteristic properties and many uses.
Define the term electronegativity.
(i) Outline two reasons why a sodium ion has a smaller radius than a sodium atom.
(ii) Explain why the ionic radius of \({{\text{P}}^{3 - }}\) is greater than the ionic radius of \({\text{S}}{{\text{i}}^{4 + }}\).
The graph below represents the successive ionization energies of sodium. The vertical axis plots log (ionization energy) instead of ionization energy to allow the data to be represented without using an unreasonably long vertical axis.
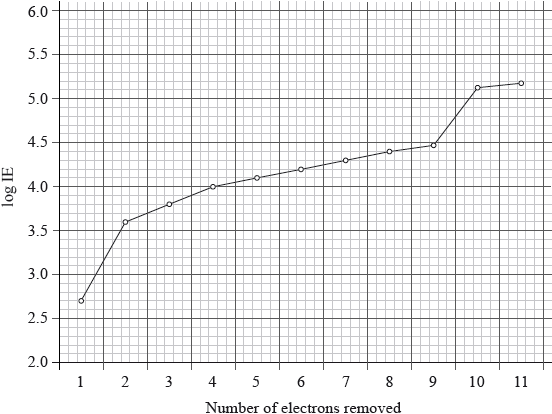
State the full electron configuration of sodium and explain how the successive ionization energy data for sodium are related to its electron configuration.
(i) Explain why the first ionization energy of aluminium is lower than the first ionization energy of magnesium.
(ii) Explain why the first ionization energy of sulfur is lower than the first ionization energy of phosphorus.
State and explain the type of reaction that takes place between \({\text{F}}{{\text{e}}^{3 + }}\) and \({{\text{H}}_{\text{2}}}{\text{O}}\) to form \({{\text{[Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) in terms of acid-base theories.
Explain why \({{\text{[Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) is coloured.
Outline the economic significance of the use of a catalyst in the Haber process which is an exothermic reaction.
Markscheme
power/strength/ability of an atom to attract electrons/shared electron pair / OWTTE;
in a (covalent) bond;
Accept the word “element” in place of “atom”.
Do not accept electron (singular).
(i) Na: 11 p, 11/ 2.8.1 \({{\text{e}}^ - }\) and \({\text{N}}{{\text{a}}^ + }\): 11 p, 10 / 2.8 \({{\text{e}}^ - }\) / same number of protons, less electrons / \({\text{N}}{{\text{a}}^ + }\) has 2 shells/energy levels, Na has 3 / OWTTE;
Na+: has greater net positive charge/same number of protons pulling smaller number of electrons;
(ii) Si4+: 10 \({{\text{e}}^ - }\) in 2 (filled) energy levels / electron arrangement 2.8 / OWTTE;
P3−: 18 \({{\text{e}}^ - }\) in 3 (filled) energy levels / electron arrangement 2.8.8, thus larger / OWTTE;
OR
\({\text{S}}{{\text{i}}^{4 + }}\): has 2 energy levels where as \({{\text{P}}^{3 - }}\) has 3/ \({{\text{P}}^{3 - }}\) has one more (filled) energy
level;
\({\text{S}}{{\text{i}}^{4 + }}\): 10 \({{\text{e}}^ - }\) in 2 energy levels where as \({{\text{P}}^{3 - }}\) has 18 \({{\text{e}}^ - }\), thus larger;
\({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{1}}}\);
Do not accept [Ne] 3s1.
first electron easy/easiest to remove / 1 electron in outermost/\({\text{n}} = 3\) energy level;
large increase between 1st and 2nd IE as electron now removed from \({\text{n}} = 2\) / next 8 electrons more difficult to remove / show (relatively) small increase as these electrons are in the same energy level/second energy level/\({\text{n}} = 2\);
large increase between 9th and 10th IE as electron now removed from n = 1 / 2
electrons very hard/most difficult to remove / innermost/lowest/closest to the nucleus/energy level/\({\text{n}} = 1\) / OWTTE;
electron 11 also comes from 1s, so shows a small increase;
(i) outer electron in Al is in 3p/p orbital/sub-shell/sub-level;
higher orbital/sub-shell / e– further from nucleus / shielded by 3s electrons;
(ii) in S, electron paired in 3p/p orbital/sub-shell/sub-level;
Accept extra stability associated with half filled p sub-shell (in P).
repulsion between paired electrons (and therefore easier to remove);
Lewis acid-base (reaction);
\({{\text{H}}_{\text{2}}}{\text{O}}\): e-pair donor, \({\text{F}}{{\text{e}}^{3 + }}\): \({{\text{e}}^ - }\) pair acceptor / \({{\text{H}}_{\text{2}}}{\text{O}}\) donates an electron pair to \({\text{F}}{{\text{e}}^{3 + }}\);
d sub-levels are split into two sets of orbitals (of different energies);
electron transitions between (d) orbitals of different energies / d-d transition(s);
transmitted (visible) light is complementary colour;
(exothermic reactions) low temperature/less energy increases ammonia yield;
(iron) catalyst used to increase rate of reaction / equilibrium reached faster / same yield but produced faster/in shorter/less time;
Examiners report
Generally, the definition of electronegativity was good, but some made the error of saying that it was the attraction of one electron only; others did not specify that it is the ability of an atom to attract a shared electron pair in a covalent bond.
Reasons why a sodium ion has a smaller radius than a sodium atom solicited incomplete answers. The answer requires the number of shells, electrons and protons of both the ion and the atom. Many candidates correctly said that \({\text{N}}{{\text{a}}^ + }\) had the same number of protons but one electron less so the pulling effect on the electrons was greater. Not many candidates gave the electronic structure or number of shells of the two ions, \({{\text{P}}^{3 - }}\) and \({\text{S}}{{\text{i}}^{4 + }}\), to explain their difference in ionic radius.
The graphical question on successive ionization energies of sodium was well answered by many. Typically, they explained how the successive ionization energies of sodium are related to its electron configuration from the data given. Most candidates realized that aluminium’s outer electron is in the 3p orbital so further from the nucleus and thus easier to ionize than magnesium. Similarly, sulfur has a paired electron in the 3p sub-shell and the repulsion between paired electrons is greater than in phosphorus which has a half filled p sub-shell.
Many candidates did not give sufficient answers to the part on transition elements. Some realised that it was a Lewis acid-base reaction where the electrons are donated by the water molecule to \({\text{F}}{{\text{e}}^{3 + }}\). Explanations given for the colour of complex ions continue to be muddled and the language used imprecise. Many wrote of “a split d orbital” rather than the d sub-level being split into two sets of orbitals (of different energies). The colour seen was often attributed to electrons emitting those wavelengths in transitions from higher energy to lower energy d orbitals rather than the transmitted visible light being the complementary colour of the one absorbed.
Few complete answers were given about economic significance of the use of a catalyst in the Haber process. A point that was missing often was that because the reaction is exothermic the forward reaction would be favoured (and the yield) if the temperature is lowered, but this would bring about a slower reaction so a catalyst is necessary to reach the equilibrium faster. However, there were misconceptions both in as far as catalysts and energetic is concerned. It was surprising to see the number of candidates who referred to activation energy but used the concept incorrectly. Few candidates established a connection with equilibrium.
Few complete answers were given about economic significance of the use of a catalyst in the Haber process. A point that was missing often was that because the reaction is exothermic the forward reaction would be favoured (and the yield) if the temperature is lowered, but this would bring about a slower reaction so a catalyst is necessary to reach the equilibrium faster. However, there were misconceptions both in as far as catalysts and energetic is concerned. It was surprising to see the number of candidates who referred to activation energy but used the concept incorrectly. Few candidates established a connection with equilibrium.
Few complete answers were given about economic significance of the use of a catalyst in the Haber process. A point that was missing often was that because the reaction is exothermic the forward reaction would be favoured (and the yield) if the temperature is lowered, but this would bring about a slower reaction so a catalyst is necessary to reach the equilibrium faster. However, there were misconceptions both in as far as catalysts and energetic is concerned. It was surprising to see the number of candidates who referred to activation energy but used the concept incorrectly. Few candidates established a connection with equilibrium.

