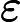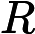DP Physics Questionbank

5.3 – Electric cells
Description
Nature of science:
Long-term risks: Scientists need to balance the research into electric cells that can store energy with greater energy density to provide longer device lifetimes with the long-term risks associated with the disposal of the chemicals involved when batteries are discarded. (4.8)
Understandings:
- Cells
- Internal resistance
- Secondary cells
- Terminal potential difference
- Emf
Applications and skills:
- Investigating practical electric cells (both primary and secondary)
- Describing the discharge characteristic of a simple cell (variation of terminal potential difference with time)
- Identifying the direction of current flow required to recharge a cell
- Determining internal resistance experimentally
- Solving problems involving emf, internal resistance and other electrical quantities
Guidance:
- Students should recognize that the terminal potential difference of a typical practical electric cell loses its initial value quickly, has a stable and constant value for most of its lifetime, followed by a rapid decrease to zero as the cell discharges completely
Data booklet reference:
International-mindedness:
- Battery storage is important to society for use in areas such as portable devices, transportation options and back-up power supplies for medical facilities
Theory of knowledge:
- Battery storage is seen as useful to society despite the potential environmental issues surrounding their disposal. Should scientists be held morally responsible for the long-term consequences of their inventions and discoveries?
Utilization:
- The chemistry of electric cells (see Chemistry sub-topics 9.2 and C.6)
Aims:
- Aim 6: experiments could include (but are not limited to): investigation of simple electrolytic cells using various materials for the cathode, anode and electrolyte; software-based investigations of electrical cell design; comparison of the life expectancy of various batteries
- Aim 8: although cell technology can supply electricity without direct contribution from national grid systems (and the inherent carbon output issues), safe disposal of batteries and the chemicals they use can introduce land and water pollution problems
- Aim 10: improvements in cell technology have been through collaboration with chemists
Directly related questions
- 16N.1.HL.TZ0.17: A 12V battery has an internal resistance of 2.0Ω. A load of variable resistance is connected...
- 17M.1.SL.TZ1.15: Two pulses are travelling towards each other. What is a possible pulse shape when the pulses...
- 17M.2.SL.TZ1.5b.i: Explain which interaction is responsible for this decay.
-
17M.3.SL.TZ2.2b:
In one experiment a student obtains the following graph showing the variation with current I of the potential difference V across the cell.
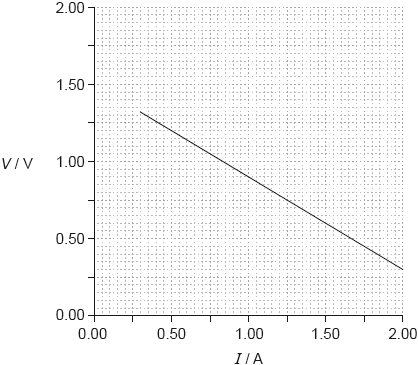
Using the graph, determine the best estimate of the internal resistance of the cell.
-
20N.1.HL.TZ0.18:
An electrical power supply has an internal resistance. It supplies a direct current to an external circuit for a time . What is the electromotive force (emf) of the power supply?
A.
B.
C.
D.
- 17N.1.SL.TZ0.19: With reference to internal energy conversion and ability to be recharged, what are...
-
17N.3.SL.TZ0.2a:
Show that the gradient of the graph is equal to .
- 17N.3.SL.TZ0.2b: State the value of the intercept on the R axis.
-
21M.2.SL.TZ2.6a:
Explain why the output potential difference to the external circuit and the output emf of the photovoltaic cell are different.
-
21M.2.SL.TZ2.6b:
Calculate the internal resistance of the photovoltaic cell for the maximum intensity condition using the model for the cell.
- 21M.1.SL.TZ1.20: For a real cell in a circuit, the terminal potential difference is at its closest to the emf...
-
21M.1.SL.TZ2.21:
Three identical resistors of resistance R are connected as shown to a battery with a potential difference of and an internal resistance of . A voltmeter is connected across one of the resistors.
What is the reading on the voltmeter?
A.
B.
C.
D.
-
18M.1.HL.TZ1.17:
When an electric cell of negligible internal resistance is connected to a resistor of resistance 4R, the power dissipated in the resistor is P.
What is the power dissipated in a resistor of resistance value R when it is connected to the same cell?
A.
B. P
C. 4P
D. 16P
- 18M.1.SL.TZ1.20: Five resistors of equal resistance are connected to a cell as shown. ...
- 18M.1.SL.TZ2.22: A cell has an emf of 4.0 V and an internal resistance of 2.0 Ω. The ideal voltmeter reads 3.2...
-
18M.2.SL.TZ2.4a:
State what is meant by the emf of a cell.
- 21N.1.SL.TZ0.21: A variable resistor is connected in series to a cell with internal resistance r as shown. The...
- 21N.1.HL.TZ0.17: A cell has an emf of 3.0 V and an internal resistance of 2.0 Ω. The cell is connected in series...
-
18M.2.HL.TZ2.4a:
State what is meant by the emf of a cell.
- 22M.2.SL.TZ2.4b.i: State the emf of the cell.
-
22M.2.SL.TZ2.4b.ii:
Deduce the internal resistance of the cell.
-
22M.2.HL.TZ2.4c.ii:
Comment on the implications of your answer to (c)(i) for cell B.
- 22M.2.HL.TZ2.4b.i: State the emf of the cell.
-
22M.2.HL.TZ2.4b.ii:
Deduce the internal resistance of the cell.
-
19M.2.HL.TZ2.4a:
The switch S is initially open. Calculate the total power dissipated in the circuit.
- 19M.2.HL.TZ2.4bi: The switch is now closed. State, without calculation, why the current in the cell will increase.
- 19M.3.SL.TZ2.1c: Outline, without calculation, how the internal resistance can be determined from this graph.
-
19M.2.HL.TZ2.4bii:
The switch is now closed. .
-
19M.2.SL.TZ1.1d:
Determine the internal resistance of the battery.
-
19M.2.SL.TZ1.1e.i:
Calculate the emf of one cell.
- 19N.1.SL.TZ0.19: The diagram shows a resistor network. The potential difference between X and Y is 8.0 V. What...