| Date | May 2018 | Marks available | 1 | Reference code | 18M.1.HL.TZ2.9 |
| Level | Higher level | Paper | Paper 1 | Time zone | Time zone 2 |
| Command term | Question number | 9 | Adapted from | N/A |
Question
Q and R are two rigid containers of volume 3V and V respectively containing molecules of the same ideal gas initially at the same temperature. The gas pressures in Q and R are p and 3p respectively. The containers are connected through a valve of negligible volume that is initially closed.
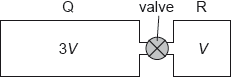
The valve is opened in such a way that the temperature of the gases does not change. What is the change of pressure in Q?
A. +p
B. \(\frac{{ + p}}{2}\)
C. \(\frac{{ - p}}{2}\)
D. –p
Markscheme
B
Examiners report
[N/A]

