| Date | November 2009 | Marks available | 2 | Reference code | 09N.3.sl.TZ0.C2 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | Explain | Question number | C2 | Adapted from | N/A |
Question
Polyvinyl chloride (PVC) and polyethene are both polymers made from crude oil.
Explain why PVC is less flexible than polyethene.
State how PVC can be made more flexible during its manufacture and explain the increase in flexibility on a molecular level.
PVC can exist in isotactic and atactic forms. Draw the structure of the isotactic form showing a chain of at least six carbon atoms.
Markscheme
C–Cl bond / molecule is polar;
stronger intermolecular/van der Waals’/London/dispersion forces/dipole-dipole attraction;
addition of plasticizers;
Allow misspelling within reason.
get between polymer chains / keeps chains further apart and reduces attraction (between the chains);
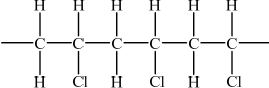 ;
;
Accept any structure with all the Cl atoms shown on the same side.
Continuation bonds at end of structure not needed.
Hydrogen atoms must be included.
Examiners report
Most answers in (a) did not mention the polarity of the C–Cl bond.
The explanation of PVC's flexibility in (b) did not usually refer to the polymer chains being pushed apart.
A significant number of candidates drew the isotactic form of polypropene instead of PVC in response to (c).

