| Date | May 2012 | Marks available | 3 | Reference code | 12M.2.hl.TZ2.10 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Deduce and State | Question number | 10 | Adapted from | N/A |
Question
Esters and amides can be produced by condensation reactions.
Under certain conditions but-2-ene can react with water to form butan-2-ol.
State the names of two organic compounds required to produce ethyl methanoate and state suitable reaction conditions.
Deduce the structure of the simplest repeating unit of the polymer formed from the reaction between 1,6-diaminohexane and hexane-1,6-dioic acid and state one use of this product.
State and explain how the rate of step II would differ if 2-chlorobutane was used instead of 2-bromobutane.
Markscheme
ethanol and methanoic acid/methanoic anhydride/methanoyl chloride;
\({{\text{H}}_2}{\text{S}}{{\text{O}}_4}/{{\text{H}}^ + }\) and heat;
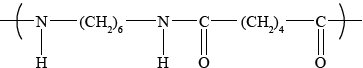
Award [1] for the correct amide link.
Award [1] if the rest of the structure is correct.
nylon fabric / clothing / ropes;
slower rate because carbon to chlorine bond stronger than carbon to bromine bond / OWTTE;
Examiners report
In part (a) most candidates correctly named both compounds but did not state both the catalyst and heat as necessary conditions for the production of ethyl methanoate.
Several candidates had difficulty in deducing the structure of the simplest repeating unit but most knew the uses of the product.
Assessment statements 10.6 and 20.5 state that reagents, conditions and equations are required. Most candidates correctly explained the effect of chlorine rather than bromine on the rate of the substitution reaction.

