| Date | May 2012 | Marks available | 3 | Reference code | 12M.3.sl.TZ2.C3 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Draw and Explain | Question number | C3 | Adapted from | N/A |
Question
Ethene can be polymerized to form high-density poly(ethene), HDPE, or low-density poly(ethene), LDPE, depending on the reaction conditions. Describe the main structural difference between HDPE and LDPE and explain how this accounts for their different properties.
(i) The repeating unit of poly(propene) has the formula:
–[–\({\text{C}}{{\text{H}}_{\text{2}}}{\text{–CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}\)–]–
Draw a section of the polymer containing five repeating units to illustrate atactic
poly(propene).
(ii) Explain why isotactic poly(propene) is tough and can be used to make car bumpers (fenders), whereas atactic poly(propene) is soft and flexible making it suitable for sealants.
Markscheme
HDPE has little branching whereas LDPE has branching/side chains / OWTTE;
HDPE is stronger/more rigid / LDPE is weaker/more flexible/resilient;
HDPE chains pack more closely together / LDPE chains pack less closely together; HDPE has stronger van der Waals’ forces / LDPE has weaker van der Waals’ forces;
(i) 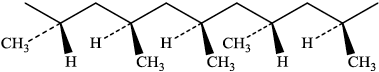 ;
;
There must be five –CH3 groups and they must be shown in random orientation.
Allow the following structure:
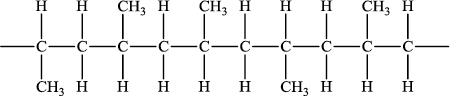
(ii) isotactic has all methyl groups oriented/pointing in the same direction/has a more regular structure / atactic has the methyl groups oriented/pointing in different directions/arranged randomly/has an irregular structure;
in isotactic the chains can pack more closely together (making it more crystalline/tough) / in atactic the chains pack less closely together (making it soft/flexible);
isotactic has stronger van der Waals’ forces / atactic has weaker van der Waals’ forces;
Examiners report
In (a) most candidates were aware of the differences between HDPE and LDPE, but often failed to fully score as they referred to intermolecular forces in a rather vague, instead of specific, manner.
Most candidates found it difficult to draw the structure of atactic poly(propene) in part (b)(i). Most candidates were very familiar with the difference in structure of isotactic and atactic poly(propene), but many failed to fully score as their responses lacked the required specificity for the intermolecular forces.

