| Date | November 2009 | Marks available | 1 | Reference code | 09N.3.hl.TZ0.F2 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Explain | Question number | F2 | Adapted from | N/A |
Question
Antioxidants can be used to prolong the shelf life of food.
\({{\text{(EDTA)}}^{4 - }}\), the ethylenediaminetetraacetate anion, is a chelate ligand with the following structure.
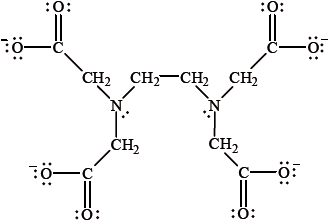
It has been found to inhibit the \({\text{F}}{{\text{e}}^{2 + }}\) catalysed oxidation of raw beef. Explain why \({{\text{(EDTA)}}^{4 - }}\) can be described as a chelate ligand.
Markscheme
hexadentate/polydentate/crab-claw (type) ligand which coordinates to the metal/iron / OWTTE;
Examiners report
In (d), very few candidates knew why \({{\text{(EDTA)}}^ - }\) can be described as a chelate ligand which again showed a clear weakness of a key chemical concept within this option.

