| Date | November 2009 | Marks available | 2 | Reference code | 09N.2.hl.TZ0.9 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | State | Question number | 9 | Adapted from | N/A |
Question
The compound \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{7}}}{\text{Cl}}\) can exhibit stereoisomerism.
The reaction between bromoethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\), and potassium cyanide is an example of a nucleophilic substitution reaction.
Draw the structural formulas of the two geometrical isomers of 1-chloro-but-2-ene.
Explain why 1-chloro-but-2-ene shows geometrical isomerism.
Draw the structural formula of one isomer of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{7}}}{\text{Cl}}\) that shows optical isomerism and identify the chiral carbon atom with an asterisk (*).
State whether this reaction is SN1 or SN2.
Explain the mechanism of the reaction using curly arrows to represent the movement of electron pairs.
The organic product obtained in part (c) (ii) can be reduced to form an amine. State an equation for the reaction, naming the catalyst involved.
Markscheme
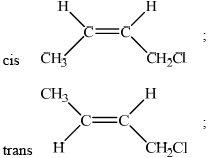
no rotation possible due to double bond/pi bond;
Accept hindered or restricted rotation.
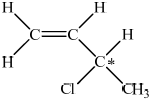
correct structural formula;
chiral carbon atom identified;
SN2;
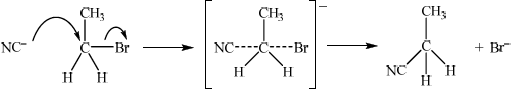
curly arrow going from \({\text{C}}{{\text{N}}^ - }\) to C;
curly arrow showing Br leaving;
Curly arrow may be represented on transition state.
representation of transition state, showing negative charge and dotted lines;
products;
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CN}} + {\text{2}}{{\text{H}}_{\text{2}}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\);
Ni / Pt / Pd;
Examiners report
The standard of organic chemistry this session was slightly better when compared to previous sessions. In part (a), some candidates missed that no rotation is possible due to the pi bond. The question required candidates to draw the two geometrical isomers of 1-chloro-but-2-ene but some candidates had drawn the isomers of 1-chloro-but-1-ene or 2-chloro-but-2-ene. Only the able candidates could draw the optical isomer of C4H7Cl and identify the chiral carbon atom.
In part (c), the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism between bromoethane and potassium cyanide proved to be a challenge as candidates continued to make the same errors as found in previous sessions, such as an incorrect placement of curly arrows, the omission of non-bonding pairs of electrons on the nucleophile and the failure to include partial bonds and an overall charge in the formula of the \({{\text{S}}_{\text{N}}}{\text{2}}\) transition state. Candidates are encouraged to show the entering and leaving groups at 180° instead of 90° on the transition state.
In part (c), the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism between bromoethane and potassium cyanide proved to be a challenge as candidates continued to make the same errors as found in previous sessions, such as an incorrect placement of curly arrows, the omission of non-bonding pairs of electrons on the nucleophile and the failure to include partial bonds and an overall charge in the formula of the \({{\text{S}}_{\text{N}}}{\text{2}}\) transition state. Candidates are encouraged to show the entering and leaving groups at 180° instead of 90° on the transition state.
In part (c), the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism between bromoethane and potassium cyanide proved to be a challenge as candidates continued to make the same errors as found in previous sessions, such as an incorrect placement of curly arrows, the omission of non-bonding pairs of electrons on the nucleophile and the failure to include partial bonds and an overall charge in the formula of the \({{\text{S}}_{\text{N}}}{\text{2}}\) transition state. Candidates are encouraged to show the entering and leaving groups at 180° instead of 90° on the transition state.

