| Date | November 2013 | Marks available | 1 | Reference code | 13N.1.sl.TZ0.3 |
| Level | SL | Paper | 1 | Time zone | TZ0 |
| Command term | Determine | Question number | 3 | Adapted from | N/A |
Question
What are the coefficients of \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\) and \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\) when the following equation is balanced using the smallest possible whole numbers?
___ \({\text{C}}{{\text{a}}_{\text{3}}}{{\text{(P}}{{\text{O}}_{\text{4}}}{\text{)}}_{\text{2}}}{\text{(s)}} + \) ___ \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} \to \) ___ \({\text{CaS}}{{\text{O}}_4}{\text{(s)}} + \) ___ \({{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}}\)
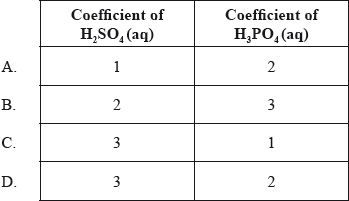
Markscheme
D
Examiners report
[N/A]
Syllabus sections
Core » Topic 1: Stoichiometric relationships » 1.1 Introduction to the particulate nature of matter and chemical change
Show 40 related questions

