| Date | November 2013 | Marks available | 2 | Reference code | 13N.3.sl.TZ0.8 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | Explain | Question number | 8 | Adapted from | N/A |
Question
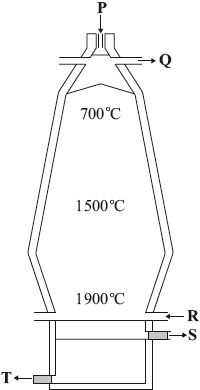
Iron ore can be reduced in a blast furnace.
The properties of a metal can be altered by alloying or heat treatment.
Explain why alloying can modify the structure and properties of a metal.
Markscheme
(alloying element(s)) atoms/ions have different size;
Allow suitable diagram.
disrupts regular/repeating (metal) lattice;
difficult for one layer to slide over another / added atoms/ions smaller than metal atoms/ions can fit into the (holes of) metal lattice disrupting bonding;
If “particles” is penalised in M1, allow “particles” in M3.
Do not award mark for different or unique properties of alloys.
Examiners report
The common errors in (a) were to give a formula rather than the name of an ore and to add it at R. Whilst part (ii) was generally answered correctly it was slightly alarming to see oxygen given from time to time. Few seemed to know the equation of the reaction primarily responsible for the temperature of 1900 °C. Slag was usually correctly identified in (c) but the equations given seldom started from the raw material, \({\text{CaC}}{{\text{O}}_{\text{3}}}\). Part (d) was often answered successfully whilst candidates were generally unable to earn both marks in (e) (i). Most understood the effect of tempering on steel.

