| Date | November 2013 | Marks available | 2 | Reference code | 13N.3.hl.TZ0.10 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Explain | Question number | 10 | Adapted from | N/A |
Question
Iron ore can be reduced in a blast furnace.
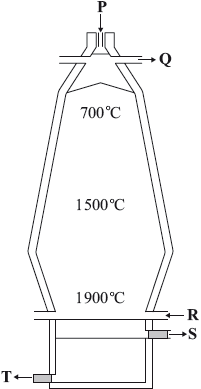
The properties of a metal can be altered by alloying or heat treatment. Explain why alloying can modify the structure and properties of a metal.
Markscheme
(alloying element(s)) atoms/ions have different size;
Allow suitable diagram.
disrupts regular/repeating (metal) lattice;
difficult for one layer to slide over another / added atoms/ions smaller than metal atoms/ions can fit into the (holes of) metal lattice disrupting bonding;
If “particles” is penalised in M1, allow “particles” in M3.
Do not award mark for different or unique properties of alloys.
Examiners report
Option C was not a popular option.
While many candidates scored the mark in 10 (a), those who did not often failed to provide the correct name for an ore. Although many identified slag, some were able to give the correct equation and others gave equations which were either incorrect or not from raw materials as asked. This question unfortunately shows that chemical equations seem not to be as well covered as expected. The answer to the question on alloys was rather disappointing and weaker than in previous sessions. The lack of subject specific vocabulary was often observed with many candidates providing answers that were clearly not addressing the question. Very few candidates were able to score even one mark on the mechanism by which the carbon chain increases in length during the manufacture of LDPE suggesting that this topic requires further attention. Many candidates were familiar with the catalyst used in the formation of HDPE although some lost the mark due to writing names that differed widely from correct one. Many were able to score at least one mark for the structure of the isotactic form of the polymer but very few drew 3D structures. Many candidates were able to score partial points when explaining why the isotactic form is more suitable for the manufacture of strong fibres but many missed the idea of chains not being able to move past each other easily (hence fibre is strong/rigid).
The part on liquid crystal displays was done with mixed results with many correct answers but still below expectations. Many candidates scored a mark for the explanation of how the addition of a LC to a cell changes what the observer sees usually from establishing the rotation of the plane of polarized light, but far too often replies were shallow with limited use of correct terminology. In the explanation of how the application of an electric filed between electrodes changes what the observer sees, many students were able to score one mark by stating that light is not transmitted but only stronger candidates included in their answers that molecules are aligned or not twisted. The question on the Ni-Cd battery was answered poorly with many candidates not even attempting it or getting the equation completely wrong and not being able to identify insolubility of the products that allows the reaction to be reversed and the cell charged. Description of the addition of small amounts of arsenic to increase the conductivity of silicon was surprising not done well and is a topic that needs closer attention.

