| Date | November 2014 | Marks available | 2 | Reference code | 14N.3.sl.TZ0.8 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | Outline and State | Question number | 8 | Adapted from | N/A |
Question
Aluminium is chemically reactive so it has to be extracted by the electrolysis of aluminium oxide dissolved in molten cryolite.
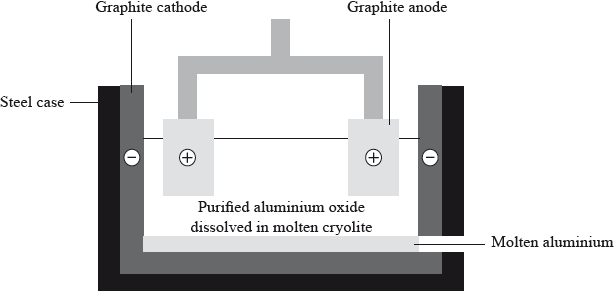
Deduce an equation for the discharge of the ions at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
(i) Outline why aluminium is alloyed with copper and magnesium when used to construct aircraft bodies.
(ii) State two properties of aluminium that make it suitable for use in overhead power cables.
Markscheme
Positive electrode (anode):
\({\text{2}}{{\text{O}}^{2 - }} \to {{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{e}}^ - }/{{\text{O}}^{2 - }} \to \frac{1}{2}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ - }/{\text{2}}{{\text{O}}^{2 - }} - {\text{4}}{{\text{e}}^ - } \to {{\text{O}}_2}{\text{(g)}}/\)
\({{\text{O}}^{2 - }} - {\text{2}}{{\text{e}}^ - } \to \frac{1}{2}{{\text{O}}_2}{\text{(g)}}\);
Allow C(s) + 2O2– \( \to \) CO2(g) + 4e–.
Negative electrode (cathode):
\({\text{A}}{{\text{l}}^{3 + }} + {\text{3}}{{\text{e}}^ - } \to {\text{Al(l)}}\);
Accept e instead of e–.
Ignore state symbols.
If correct equations shown at wrong electrodes, award [1 max].
(i) harder/stronger (than pure aluminium);
(ii) Award [1] for any two of:
good conductor of electricity;
resists corrosion;
Do not allow rusting.
low density;
Do not allow lighter/light mass/light weight.
ductile;
Do not allow malleable.
Examiners report
In (a), most were able to write the correct half-equation for the cathode though incorrect states were commonly seen, e.g. (aq). The anode half-equation was not well known.
Both parts of (b) were well done. In (ii), incorrect answers included malleability and light mass.

