| Date | May 2018 | Marks available | 2 | Reference code | 18M.3.sl.TZ1.9 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Identify | Question number | 9 | Adapted from | N/A |
Question
Greenhouse gases absorb infrared radiation.
Identify one naturally occurring greenhouse gas, other than carbon dioxide or water vapour, and its natural source.
Formulate an equation that shows how aqueous carbon dioxide produces hydrogen ions, H+(aq).
The concentrations of oxygen and nitrogen in the atmosphere are much greater than those of greenhouse gases. Outline why these gases do not absorb infrared radiation.
Markscheme
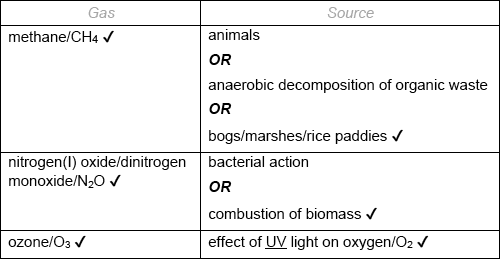
Accept “nitrous oxide”.
Accept “electrical discharges/lightning”.
[2 marks]
CO2(aq) + H2O(l) \( \rightleftharpoons \) H+(aq) + HCO3–(aq)
OR
CO2(aq) + H2O(l) \( \rightleftharpoons \) H2CO3(aq) AND H2CO3(aq) \( \rightleftharpoons \) H+(aq) + HCO3–(aq)
Accept CO2(aq) + H2O(l) \( \rightleftharpoons \) 2H+(aq) + CO32–(aq).
Accept equations with single arrow.
[1 mark]
no change in polarity/dipole «moment when molecule vibrates»
Do not accept “non-polar” or “no dipole moment” – idea of change must be there.
[1 mark]

