| Date | November 2010 | Marks available | 3 | Reference code | 10N.3.sl.TZ0.A2 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | Explain | Question number | A2 | Adapted from | N/A |
Question
Infrared (IR) spectroscopy is widely used as a technique in analytical chemistry.
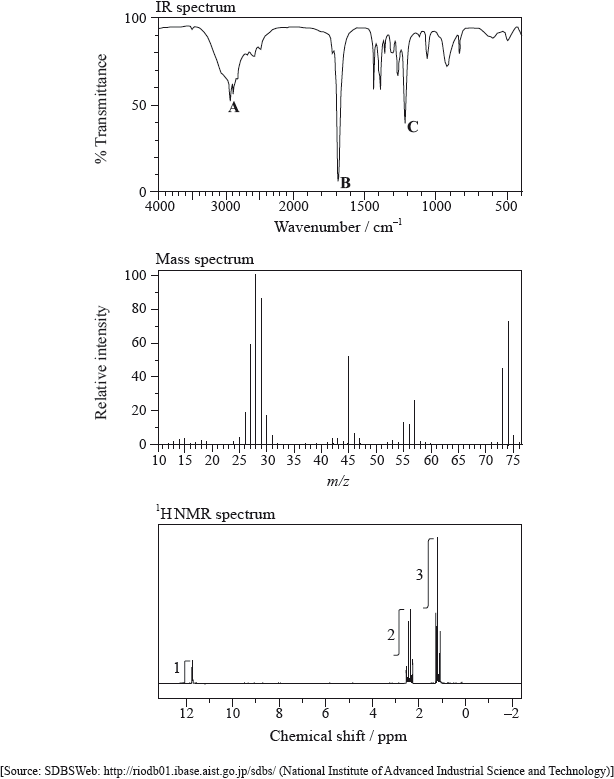
The IR spectrum, mass spectrum and \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of an unknown compound, X, of molecular formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\) are as follows.
Explain what happens at a molecular level during the absorption of IR radiation by carbon dioxide, CO2.
(i) Identify the bonds responsible for the peaks A, B and C in the IR spectrum of X.
A:
B:
C:
(ii) In the mass spectrum of X, deduce which ions the m/z values at 74, 45 and 29 correspond to.
m/z = 74:
m/z = 45:
m/z = 29:
(iii) Identify the peak at 11.73 ppm in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum.
(iv) Deduce the structure of X.
Markscheme
change in bond length / bond stretching / asymmetric stretch;
change in bond angle / bending (of molecule);
Allow [1 max] for only stating vibrations.
induces molecular polarity/dipole moment / OWTTE;
(i) A: O–H
B: C=O
C: C–O
Award [2] for three correct, [1] for two correct.
(ii) m/z = 74: \({{\text{C}}_2}{{\text{H}}_5}{\text{COO}}{{\text{H}}^ + }\) / \({{\text{C}}_3}{{\text{H}}_6}{\text{O}}_2^ + \);
m/z = 45: \({\text{COO}}{{\text{H}}^ + }\);
m/z = 29: \({{\text{C}}_2}{\text{H}}_5^ + \);
Penalize missing + charge once only.
Do not award mark for m/z = 29: CHO+.
(iii) –COOH
Accept –OH.
(iv) \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH}}\) / \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{O}}_2}{\text{H}}\);
More detailed structural formula may be given.
Examiners report
In (b) the main misconception stated by candidates was that non-polar compounds do not absorb infrared radiation. Most candidates scored a mark for vibrations, but many misunderstood the difference between symmetric and asymmetric stretching.
Part (c)(i) was well answered by the great majority of candidates; giving C–H bond instead of O–H for A was a popular incorrect answer. In (ii) the most common mistake was missing the + sign. Most candidates answered (iii) and (iv) correctly.

