| Date | May 2009 | Marks available | 3 | Reference code | 09M.3.sl.TZ1.A1 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Explain | Question number | A1 | Adapted from | N/A |
Question
Explain what occurs at a molecular level during the absorption of infrared (IR) radiation by the sulfur dioxide molecule, \({\text{S}}{{\text{O}}_{\text{2}}}\).
Consider the IR spectra of the following three compounds.
\[\begin{array}{*{20}{l}} {{\text{A}} = {\text{C}}{{\text{H}}_{\text{3}}}{{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}}_{\text{3}}}{\text{COOH}}} \\ {{\text{B}} = {\text{C}}{{\text{H}}_{\text{3}}}{\text{COOC(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}} \\ {{\text{C}} = {{{\text{(C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{)}}}_{\text{3}}}{\text{COH}}} \end{array}\]
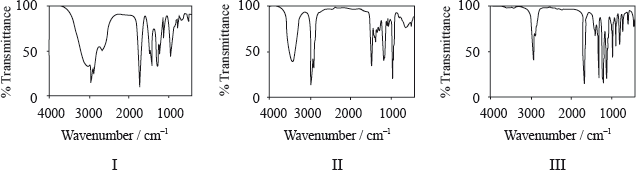
Determine which IR spectrum corresponds to each compound A, B and C. Explain your reasoning. IR data can be found in Table 17 of the Data Booklet.
Markscheme
(O–S–O) bond angle changes;
(S–O) bond (length) stretches;
Allow [1] for S–O bond vibrations if neither of the above points are scored.
A is Spectrum I and B is Spectrum III and C is Spectrum II;
A Spectrum I:
only spectrum with a (broad) peak in the range \({\text{2500–3300 (c}}{{\text{m}}^{ - 1}}{\text{)}}\) corresponding to the carboxylic acid functional group / –OH in carboxylic acid / H-bonding in carboxylic acid (so must be a carboxylic acid);
B Spectrum III:
peak in the range \({\text{1700–1750 (c}}{{\text{m}}^{ - 1}}{\text{)}}\) corresponding to the carbonyl/C=O group;
but no peak for O–H/no peak at \({\text{2500–3300 (c}}{{\text{m}}^{ - 1}}{\text{)}}\) or \({\text{3200–3600 (c}}{{\text{m}}^{ - 1}}{\text{)}}\);
C Spectrum II:
peak in the range \({\text{3200–3600 (c}}{{\text{m}}^{ - 1}}{\text{)}}\) corresponding to the alcohol functional group/OH / the only one without a peak at \({\text{1700–1750 (c}}{{\text{m}}^{ - 1}}{\text{)}}\) corresponding to a carbonyl/C=O group;
Examiners report
For part (b) candidates often missed discussing the change of dipole moment.
Part (d) illustrated candidates’ ability at linking wave numbers from IR spectra to correct bonds but they did not always provide adequate explanations for their choices.

