| Date | November 2011 | Marks available | 1 | Reference code | 11N.2.sl.TZ0.7 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | State | Question number | 7 | Adapted from | N/A |
Question
Consider the following reactions.
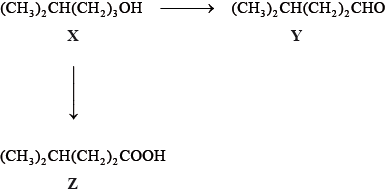
An important environmental consideration is the appropriate disposal of cleaning solvents. An environmental waste treatment company analysed a cleaning solvent, J, and found it to contain the elements carbon, hydrogen and chlorine only. The chemical composition of J was determined using different analytical chemistry techniques.
Combustion Reaction:
Combustion of 1.30 g of J gave 0.872 g \({\text{C}}{{\text{O}}_{\text{2}}}\) and 0.089 g \({{\text{H}}_{\text{2}}}{\text{O}}\).
Precipitation Reaction with AgNO3(aq):
0.535 g of J gave 1.75 g AgCl precipitate.
One example of a homologous series is the alcohols. Describe two features of a homologous series.
The IUPAC name of X is 4-methylpentan-1-ol. State the IUPAC names of Y and Z.
Y:
Z:
State the reagents and reaction conditions used to convert X to Y and X to Z.
X to Y:
X to Z:
Z is an example of a weak acid. State what is meant by the term weak acid.
Discuss the volatility of Y compared to Z.
Determine the percentage by mass of carbon and hydrogen in J, using the combustion data.
Determine the percentage by mass of chlorine in J, using the precipitation data.
The molar mass was determined to be \({\text{131.38 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Deduce the molecular formula of J.
Markscheme
same functional group;
successive/neighbouring members differ by \({\text{C}}{{\text{H}}_{\text{2}}}\);
same general formula;
similar chemical properties;
gradation in physical properties;
Y: 4-methylpentanal;
Z: 4-methylpentanoic acid;
Award [1] if student has correct endings for both molecules but has used incorrect stem.
For both reactions reagents:
named suitable acidified oxidizing agent;
Suitable oxidizing agents are potassium dichromate(VI)/K2Cr2O7 / sodium dichromate(VI)/Na2Cr2O7 / dichromate/Cr2O72– / potassium manganate(VII)/potassium permanganate/KMnO4 / permanganate/manganate(VII)/MnO4–.
Accept H+/H2SO4 instead of sulfuric acid and acidified.
Allow potassium dichromate or sodium dichromate (i.e. without (VI)) or potassium manganate (i.e. without (VII).
Conditions:
distillation for X to Y and reflux for X to Z;
Award [1] if correct reagents and conditions identified for one process only.
acid partially dissociates/ionizes;
Y more volatile than Z;
hydrogen bonding in carboxylic acid/Z;
Accept converse argument.
\(\left( {\left( {\frac{{2 \times 1.01}}{{18.02}}} \right)(0.089) = } \right){\text{ }}1.0 \times {10^{ - 2}}{\text{ g H}}\) and \(\left( {\left( {\frac{{12.01}}{{44.01}}} \right)(0.872) = } \right){\text{ }}2.38 \times {10^{ - 1}}{\text{ g C}}\);
\(\left( {\left( {\frac{{0.238}}{{1.30}}} \right)(100) = } \right){\text{ }}18.3\% {\text{ C}}\);
\(\left( {\frac{{1.0 \times {{10}^{ - 2}}}}{{1.30}}} \right)(100) = 0.77\% {\text{ H}}\);
Award [3] for correct final answer of 18.3% C and 0.77% H without working.
Allow whole numbers for molar masses.
\(\left( {(1.75)\left( {\frac{{35.45}}{{143.32}}} \right) = } \right){\text{ }}0.433{\text{ g (Cl)}}\) and \(\left( {\left( {\frac{{0.433}}{{0.535}}} \right)(100) = } \right){\text{ }}80.9\% {\text{ (Cl)}}\);
Allow whole numbers for molar masses.
\(\left( {\frac{{18.3}}{{12.01}}} \right) = 1.52{\text{ mol C}}\) and \(\left( {\frac{{0.77}}{{1.01}}} \right) = 0.76{\text{ mol H}}\) and \(\left( {\frac{{80.9}}{{35.45}}} \right) = 2.28{\text{ mol Cl}}\);
Allow whole numbers for atomic masses.
Empirical formula \( = {{\text{C}}_2}{\text{HC}}{{\text{l}}_3}\);
Award [2] for correct empirical formula without working.
\({M_{\text{r}}} = (24.02 + 1.01 + 106.35) = 131.38\), so molecular formula is \({{\text{C}}_2}{\text{HC}}{{\text{l}}_3}\);
Award [3] for correct final answer without working.
Allow whole numbers for atomic masses.
Examiners report
Part (a) which asked for a description of a homologous series was generally very well answered.
1 out of 2 marks were commonly awarded, as students had the incorrect prefix or made errors such as 4-methylpentan-1-al instead of 4-methylpentanal.
Most candidates knew the reagents for the conversions of the alcohol but only the best candidates also knew the conditions.
Explanations of a weak acid were well done.
Explanations of volatility were well done.
Part (d) was a moles calculation based on experimental data, and was done very well by some of those that attempted it. However many candidates could not get through it and some left it blank.
Part (d) was a moles calculation based on experimental data, and was done very well by some of those that attempted it. However many candidates could not get through it and some left it blank.
Part (d) was a moles calculation based on experimental data, and was done very well by some of those that attempted it. However many candidates could not get through it and some left it blank.

