| Date | May 2010 | Marks available | 1 | Reference code | 10M.1.SL.TZ1.11 |
| Level | Standard level | Paper | Paper 1 | Time zone | Time zone 1 |
| Command term | Question number | 11 | Adapted from | N/A |
Question
A gas is contained in a cylinder by a piston.
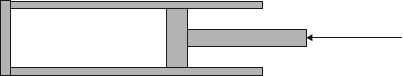
The gas is compressed rapidly by moving the piston in the direction shown. The best explanation for the resulting increase in temperature of the gas is that the molecules of the gas gain kinetic energy
A. from the moving piston.
B. by colliding more frequently with each other.
C. by being pushed closer together.
D. by colliding more frequently with the walls of the cylinder.
Markscheme
A
Examiners report
[N/A]
Syllabus sections
Show 101 related questions
- 17N.3.SL.TZ0.8d: Using your sketched graph in (b), identify the feature that shows that net work is done by...
- 17N.3.SL.TZ0.8c: The initial temperature of the gas is 290 K. Calculate the temperature of the gas at the...
- 17N.3.SL.TZ0.8b: Using the axes, sketch the three-step cycle.
- 17N.3.SL.TZ0.8a: Show that the volume of the gas at the end of the adiabatic expansion is approximately 5.3 x...
- 17N.3.SL.TZ0.10d: The Hubble Space reflecting telescope has a Cassegrain mounting. Outline the main optical...
- 17N.3.SL.TZ0.10c: The final image of the Moon is observed through the eyepiece. The focal length of the...
- 17N.3.SL.TZ0.10b: When the Earth-Moon distance is 363 300 km, the Moon is observed using the telescope. The...
- 17N.3.SL.TZ0.10a: Complete the diagram, with a Newtonian mounting, continuing the two rays to show how they...
- 17M.3.SL.TZ2.7b: State and explain at which point in the cycle ABCA the entropy of the gas is the largest.
- 17M.3.SL.TZ2.7a.iv: Determine the efficiency of the heat engine.
- 17M.3.SL.TZ2.7a.iii: Show that the thermal energy removed from the gas for the change BC is approximately 330 J.
- 17M.3.SL.TZ2.7a.ii: Show that the temperature of the gas at C is 386 K.
- 17M.3.SL.TZ2.7a.i: Justify why the thermal energy supplied during the expansion AB is 416 J.
- 17M.3.SL.TZ1.6e: State a reason why a Carnot cycle is of little use for a practical heat engine.
- 17M.3.SL.TZ1.6d.ii: The volume at C is 2.90 × 10–3\(\,\)m3. Calculate the temperature at C.
- 17M.3.SL.TZ1.6d.i: Show that \({P_B}V_B^{\frac{5}{3}} = nR{T_C}V_C^{\frac{2}{3}}\)
- 17M.3.SL.TZ1.6c.ii: The volume at B is 2.30 × 10–3\(\,\)m3. Determine the pressure at B.
- 17M.3.SL.TZ1.6c.i: Determine the temperature of the gas at A.
- 17M.3.SL.TZ1.6b: Identify the two isothermal processes.
- 17M.3.SL.TZ1.6a: State what is meant by an adiabatic process.
- 16N.3.SL.TZ0.10d: Discuss the production of waste heat by the power plant with reference to the first law and...
- 16N.3.SL.TZ0.10c: The nuclear power plant works at 71.0% of the Carnot efficiency. The power produced is 1.33...
- 16N.3.SL.TZ0.10b: Explain, with a reason, why a real nuclear power plant operating between the stated...
- 16N.3.SL.TZ0.10a: Calculate the Carnot efficiency of the nuclear power plant.
- 16M.3.SL.TZ0.8b: (i) State the change in entropy of a gas for the adiabatic expansion from A to D. (ii)...
- 16M.3.SL.TZ0.8a: (i) Calculate the temperature at C. (ii) Calculate the change in internal energy for...
- 15M.1.HL.TZ2.8: An ideal gas undergoes adiabatic expansion from state X to a new state of volume V. During...
- 15M.1.HL.TZ2.9: A block of ice at 0°C is placed on a surface and allowed to melt completely to give water at...
- 15M.2.SL.TZ1.3b: Three ice cubes at a temperature of 0°C are dropped into a container of water at a...
- 15M.2.HL.TZ1.4a: Estimate the total work done in the cycle.
- 15M.2.HL.TZ1.4b: The change AB is isothermal and occurs at a temperature of 420K. Calculate the number of...
- 15M.2.HL.TZ1.4c: Identify and explain the change, if any, in the entropy of the gas when it has completed one...
- 15M.2.HL.TZ1.7b: Three ice cubes at a temperature of 0ºC are dropped into a container of water at a...
- 15M.2.HL.TZ2.7e: For the cycle identify, with the letter I, an isochoric (isovolumetric) change.
- 15M.2.HL.TZ2.7f: The temperature at point X is 310 K. Calculate the temperature at point Y.
- 15M.2.HL.TZ2.7g: The shaded area WXYZ is 610 J. The total thermal energy transferred out of the gas in one...
- 15M.2.HL.TZ2.7h: The work done on the gas during the adiabatic compression XY is 210 J. Determine the change...
- 14M.1.HL.TZ1.12: The pressure–volume (P–V) graph shows an adiabatic compression of a fixed mass of an ideal...
- 14M.1.HL.TZ2.12: Which process will increase the entropy of the local surroundings? A. The melting of a block...
- 14M.2.HL.TZ1.7e: (i) State what is meant by an isothermal process. (ii) Show that process AB is isothermal.
- 14M.2.HL.TZ1.7f: State the nature of process BC.
- 14M.2.HL.TZ1.7h: Explain why it is not possible for this engine, operating in this cycle, to be 100% efficient.
- 14M.2.HL.TZ1.7g: During the cycle ABCD, the net work done by the gas is 550J. Calculate the net thermal energy...
- 15N.1.HL.TZ0.9: The graph shows how the volume of a system varies with pressure during a cycle ABCA. What...
- 15N.1.HL.TZ0.10: A system consists of a refrigerator with its door open operating in a thermally insulated...
- 15N.2.HL.TZ0.4a: Calculate the temperature of the gas at point C.
- 15N.2.HL.TZ0.4b: Compare, without any calculation, the work done and the thermal energy supplied along route...
- 14N.1.HL.TZ0.9: Which of the following can be deduced from the second law of thermodynamics? A. Thermal...
- 14N.2.HL.TZ0.8e: The graph shows how the pressure \(P\) of a fixed mass of gas varies with volume \(V\). The...
- 14N.2.HL.TZ0.8f: Determine the work done during the change represented by line A.
- 14N.2.HL.TZ0.8g: Outline, with reference to the first law of thermodynamics, the direction of change in...
- 14M.2.HL.TZ2.9b: State the nature of the change in the gas that takes place during process BC in the cycle.
- 14M.2.HL.TZ2.9a: At point A in the cycle, the fuel-air mixture is at 18 °C. During process AB, the gas is...
- 14M.2.HL.TZ2.9c: Process CD is an adiabatic change. Discuss, with reference to the first law of...
- 14M.2.HL.TZ2.9d: Explain how the diagram can be used to calculate the net work done during one cycle.
- 11N.1.HL.TZ0.11: The entropy of a system is a measure of the system’s A. total energy only.B. degree of...
- 12N.1.HL.TZ0.12: Water in a container freezes. Which of the following correctly describes the change in...
- 12N.1.HL.TZ0.10: The diagram shows a P–V cycle for a particular gas. In which of the following changes is...
- 12N.1.HL.TZ0.11: An ideal gas expands adiabatically. What energy change is true for the gas? A. It gains...
- 13N.1.HL.TZ0.8: The graph shows the variation of pressure P with volume V of an ideal gas during a...
- 13N.1.HL.TZ0.9: A piece of ice melts at constant temperature. Which of the following gives the correct change...
- 13M.1.HL.TZ1.9: Which of the following statements is consistent with the second law of thermodynamics? A....
- 13M.1.HL.TZ1.8: A fixed mass of gas is compressed in a very short period of time. Which of the following...
- 12M.1.HL.TZ1.8: The entropy of a system A. will decrease if the system’s temperature is increased. B. is...
- 12M.1.HL.TZ1.7: In the P–V diagram below, which line could represent an adiabatic change for an ideal gas?
- 13M.2.HL.TZ1.12b: The graph shows how the pressure P of a sample of a fixed mass of an ideal gas varies with...
- 13M.2.HL.TZ1.12a: With respect to a gas, explain the meaning of the terms thermal energy and internal energy.
- 11M.1.HL.TZ2.12: The diagram shows the...
- 11M.1.HL.TZ2.11: During an adiabatic...
- 12M.1.HL.TZ2.12: The following statement refers to question 11 and question 12. A gas is contained in a...
- 13M.1.HL.TZ2.11: An isolated system consists of a block of ice floating in a glass of water. The ice melts...
- 13M.1.HL.TZ2.10: The graph shows the variation of pressure P with volume V for a gas undergoing an adiabatic...
- 11M.2.HL.TZ2.5b: ...
- 11M.2.HL.TZ2.5c: ...
- 11M.2.HL.TZ2.5a: ...
- 12M.2.HL.TZ1.10a: State which of the processes is isothermal, isochoric (isovolumetric) or isobaric. Process...
- 12M.2.HL.TZ1.10b: The temperature of the gas at A is 300K. Calculate the temperature of the gas at B.
- 12M.2.HL.TZ1.10c: The increase in internal energy of the gas during process AB is 4100J. Determine the heat...
- 12M.2.HL.TZ1.10d: The gas is compressed at constant temperature. Explain what changes, if any, occur to the...
- 12M.2.HL.TZ2.8a: A gas undergoes a thermodynamic cycle. The P–V diagram for the cycle is shown below. In...
- 12M.2.HL.TZ2.8c: Estimate the total work done in the cycle.
- 12M.2.HL.TZ2.8b: With reference to the first law of thermodynamics, explain for the change of state A to...
- 11N.2.HL.TZ0.11c: Explain, with reference to the first law of thermodynamics, and without further calculation,...
- 11N.2.HL.TZ0.11a: Using data from the graph above, identify which gas, A or B, undergoes the isothermal expansion.
- 11N.2.HL.TZ0.11b: Using the graph opposite, estimate the difference in work done by each gas.
- 12N.2.HL.TZ0.6c: The piston is now pushed in slowly so that the compression is isothermal. Discuss the entropy...
- 12N.2.HL.TZ0.6a: Calculate the number of moles of air in the cylinder.
- 12N.2.HL.TZ0.6b: The cork leaves the toy after the air is compressed to a pressure of 1.9×105Pa and a volume...
- 13N.2.HL.TZ0.4c: The gas in (b) is kept in the cylinder by a freely moving piston. The gas is now heated at...
- 13N.2.HL.TZ0.4d: After heating, the gas is compressed rapidly to its original volume in (b). Outline why this...
- 11M.1.HL.TZ1.8: Which of the following is equivalent to the principle of energy conservation? A. Newton’s...
- 11M.1.HL.TZ1.9: An ideal gas undergoes the thermodynamic changes represented in the P –V diagram below...
- 11M.2.HL.TZ1.4c: The gas is isothermally compressed from state B back to state A. (i) Using the P–V diagram...
- 11M.2.HL.TZ1.4a: Calculate the temperature of the gas in state B.
- 11M.2.HL.TZ1.4b: (i) Calculate the work done by the gas in expanding from state A to state B. (ii) Determine...
- 09M.1.HL.TZ1.12: Which of the following correctly describes the entropy changes of the water molecules and the...
- 09M.1.HL.TZ1.11: A positive amount of thermal energy \(Q\) is transferred to an ideal gas from its...
- 10M.1.HL.TZ1.14: The diagram shows the pressure \(p\) and volume \(V\) relationship for one cycle of operation...
- 10N.1.HL.TZ0.10: An ideal gas expands isothermally from a state X to a new state of volume \(V\). The work...
- 09N.1.HL.TZ0.13: The graph below shows the variation of the pressure \(p\) with volume \(V\) of an ideal gas...
- 10N.2.HL.TZ0.B3d: (i) State the meanings of \(Q\) and \(W\). \(Q\): \(W\): (ii) Describe how the...

