| Date | May 2009 | Marks available | 2 | Reference code | 09M.2.sl.TZ2.1 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Comment | Question number | 1 | Adapted from | N/A |
Question
Aspirin, one of the most widely used drugs in the world, can be prepared according to the equation given below.
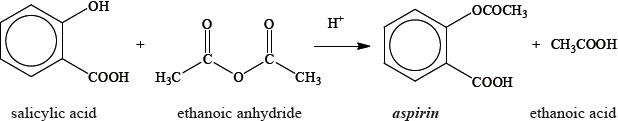
A student reacted some salicylic acid with excess ethanoic anhydride. Impure solid aspirin was obtained by filtering the reaction mixture. Pure aspirin was obtained by recrystallization. The following table shows the data recorded by the student.

State the names of the three organic functional groups in aspirin.
Determine the amount, in mol, of salicylic acid, \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{(OH)COOH}}\), used.
Calculate the theoretical yield, in g, of aspirin, \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{(OCOC}}{{\text{H}}_{\text{3}}}{\text{)COOH}}\).
Determine the percentage yield of pure aspirin.
State the number of significant figures associated with the mass of pure aspirin obtained, and calculate the percentage uncertainty associated with this mass.
Another student repeated the experiment and obtained an experimental yield of 150%. The teacher checked the calculations and found no errors. Comment on the result.
The following is a three-dimensional computer-generated representation of aspirin.
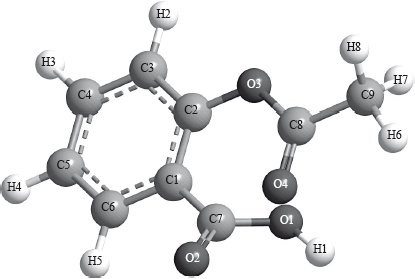
A third student measured selected bond lengths in aspirin, using this computer program and reported the following data.
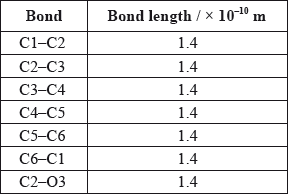
The following hypothesis was suggested by the student: “Since all the measured carbon-carbon bond lengths are equal, all the carbon-oxygen bond lengths must also be equal in aspirin. Therefore, the C8–O4 bond length must be 1.4 \( \times \) 10–10 m”. Comment on whether or not this is a valid hypothesis.
The other product of the reaction is ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\). Define an acid according to the Brønsted-Lowry theory and state the conjugate base of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\).
Brønsted-Lowry definition of an acid:
Conjugate base of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\):
Markscheme
carboxylic acid / carboxyl;
ester;
Do not allow carbonyl / acid / ethanoate / formula(–COOH).
aryl group / benzene ring / phenyl;
\({M_{\text{r}}}{\text{(}}{{\text{C}}_7}{{\text{H}}_6}{{\text{O}}_3}{\text{)}} = {\text{138.13}}\);
\(n = \left( {\frac{{3.15}}{{138.13}} = } \right){\text{ }}2.28 \times {10^{ - 2}}{\text{ (mol)}}\);
Award [2] for the correct final answer.
\({M_{\text{r}}}{\text{(}}{{\text{C}}_9}{{\text{H}}_8}{{\text{O}}_4}{\text{)}} = 180.17\);
\(m = (180.17 \times 2.28 \times {10^{ - 2}} = ){\text{ }}4.11{\text{ (g)}}\);
Accept range 4.10–4.14
Award [2] for the correct final answer.
\({\text{(percentage yield}} = \frac{{2.50}}{{4.11}} \times 100 = ){\text{ }}60.8\% \);
Accept 60–61%.
3;
\({\text{(percentage uncertainty }} = \frac{{0.02}}{{2.50}} \times 100 = {\text{) }}0.80\% \);
Allow 0.8%
sample contaminated with ethanoic acid / aspirin not dry / impure sample;
Accept specific example of a systematic error.
Do not accept error in reading balance/weighing scale.
Do not accept yield greater than 100%.
hypothesis not valid/incorrect;
Accept any of the following for the second mark
C–O and C=O bond lengths will be different;
C2–O3 bond is longer than C8–O4 bond;
C8–O4 bond shorter than C2–O3 bond;
a CO single bond is longer than a CO double bond;
Accept C8–O4 is a double bond hence shorter.
Brønsted-Lowry definition of an acid
proton/H+/hydrogen ion donor;
Conjugate base of CH3COOH
\({\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - }{\text{/C}}{{\text{H}}_3}{\text{CO}}_2^ - \);
Do not accept C2H3O2–/ethanoate.
Examiners report
In (a) Some candidates gave the correct three names of the functional groups; however some candidates gave answers such as alkene, ketone, aldehyde, ether, and carbonyl.
Candidates did not have problems determining the number of moles of salicylic acid used in (b) (i), although a few gave the answer with one significant digit only.
For (ii) the majority of candidates correctly used the value obtained in (i) to calculate the theoretical yield of aspirin.
In (iii) the percentage yield was calculated correctly in most cases.
The calculation of the percentage uncertainty (part (iv) proved to be a little more difficult, but many candidates gave the correct answer of 0.80%.
Part (v) was correctly answered by only a few candidates who stated that aspirin was contaminated or that the aspirin was not dry.
Nearly all the candidates correctly stated that the suggested hypothesis was not valid in (vi), giving the right reasons.
In (vii) most candidates gave the correct definition of an acid according to Brønsted-Lowry theory, although a few defined the acid according to Lewis theory. The conjugate base of the ethanoic acid was not always correct.

