| Date | May 2009 | Marks available | 2 | Reference code | 09M.2.sl.TZ1.6 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Explain and List | Question number | 6 | Adapted from | N/A |
Question
Predict the shape and bond angles for the following species:
Ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\), is a weak acid.
Draw the Lewis structures for carbon monoxide, CO, carbon dioxide, \({\text{C}}{{\text{O}}_{\text{2}}}\) and methanol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\).
List, with an explanation, the three compounds in order of increasing carbon to oxygen bond length (shortest first).
\({\text{C}}{{\text{O}}_{\text{2}}}\)
\({\text{CO}}_3^{2 - }\)
\({\text{BF}}_4^ - \)
Define a Brønsted-Lowry acid.
Deduce the two acids and their conjugate bases in the following reaction:
\[{{\text{H}}_2}{\text{O(l)}} + {\text{N}}{{\text{H}}_3}{\text{(aq)}} \rightleftharpoons {\text{O}}{{\text{H}}^ - }{\text{(aq)}} + {\text{NH}}_4^ + {\text{(aq)}}\]
Define the term weak acid and state the equation for the reaction of ethanoic acid with water.
Vinegar, which contains ethanoic acid, can be used to clean deposits of calcium carbonate from the elements of electric kettles. State the equation for the reaction of ethanoic acid with calcium carbonate.
Markscheme
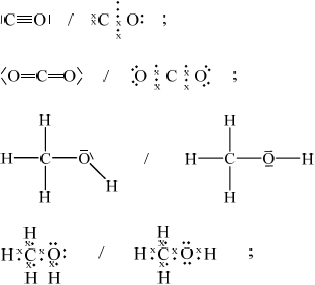
All outer electron pairs must be shown for mark in each case.
Accept electrons shown as all rather than \( \bullet \) and x.
\({\text{CO}} < {\text{C}}{{\text{O}}_{\text{2}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\);
triple bonds are shorter than double bonds which are shorter than single bonds / the more pairs of electrons that are shared the stronger the attracting so the shorter the bond / OWTTE;
The order must be correct to gain the second marking point unless ECF from (a).
\({\text{(C}}{{\text{O}}_{\text{2}}}{\text{)}}\)linear;
180°;
\({\text{(CO}}_3^{2 - }{\text{)}}\) trigonal planar/triangular planar;
120°;
\({\text{(BF}}_4^ - {\text{)}}\) tetrahedral;
109.5° / 109° / 109° \(28'\);
donates a proton / \({{\text{H}}^ + }\) ion;
\[\begin{array}{*{20}{c}} {{\text{(acid)}}}&{{\text{(conjugate base)}}} \\ {{{\text{H}}_{\text{2}}}{\text{O}}}&{{\text{O}}{{\text{H}}^ - }{\text{;}}} \\ {{\text{NH}}_4^ + }&{{\text{N}}{{\text{H}}_{\text{3}}}{\text{;}}} \end{array}\]
[1 max] if all four acids and bases given but not clearly paired.
partially dissociated or ionized;
\({\text{C}}{{\text{H}}_3}{\text{COOH}} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - } + {{\text{H}}_3}{{\text{O}}^ + }/{\text{C}}{{\text{H}}_3}{\text{COOH}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - } + {{\text{H}}^ + }\);
\( \rightleftharpoons \) required for mark.
\({\text{2C}}{{\text{H}}_3}{\text{COOH}} + {\text{CaC}}{{\text{O}}_3} \to {\text{Ca(C}}{{\text{H}}_3}{\text{COO}}{{\text{)}}_2} + {\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}}\)
Award [1] for correct reactants and products and [1] for balancing.
Examiners report
This was, by far, the most popular choice of question in Section B.
Part (a)(i) was well answered, though the weaker candidates often drew a double bond in carbon monoxide or missed out lone pairs.
These errors then gave rise to problems in attempting to answer (a)(ii).
The better candidates scored all six marks for Part (b), the weaker candidates commonly giving the correct names more often than the correct angles.
The better candidates scored all six marks for Part (b), the weaker candidates commonly giving the correct names more often than the correct angles.
The better candidates scored all six marks for Part (b), the weaker candidates commonly giving the correct names more often than the correct angles.\[28'\]
In Part (c) the definition was generally well answered and the acids and bases were usually correctly identified though not always paired as asked for in the question.
In Part (c) the definition was generally well answered and the acids and bases were usually correctly identified though not always paired as asked for in the question.
In the final equation it was rare to see a correct formula for calcium ethanoate, and even when present, the equation was not usually balanced.
In the final equation it was rare to see a correct formula for calcium ethanoate, and even when present, the equation was not usually balanced.

