| Date | May 2010 | Marks available | 2 | Reference code | 10M.3.hl.TZ1.D3 |
| Level | HL | Paper | 3 | Time zone | TZ1 |
| Command term | Describe | Question number | D3 | Adapted from | N/A |
Question
Paroxetine, whose structure is shown below, is a drug prescribed to people suffering from mental depression.
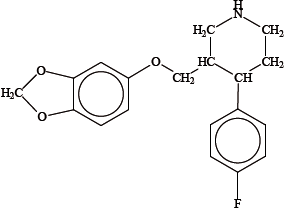
Identify the two chiral carbon atoms in the structure above with an asterisk (*).
Explain, with an example, the importance of chirality in drug action.
Describe the use of chiral auxiliaries to synthesize the desired enantiomer of a drug.
Markscheme
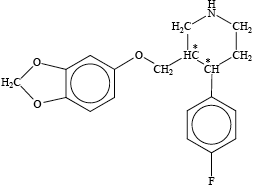
Award [1] for each correctly placed asterisk.
different enantiomers can cause different (physiological) effects in the body;
thalidomide – one isomer prevented morning sickness, the other caused fetal abnormalities / ibuprofen – one isomer is more effective than the other / DOPA – one isomer helps manage Parkinson¨disease, the other has no physiological effects;
Accept other correct examples.
chiral auxiliaries are themselves chiral;
attach to the non-chiral molecule (to enable the desired enantiomer to be formed);
after the desired enantiomer is formed the chiral auxiliary is removed/recycled;
Examiners report
The better candidates could identify the chiral carbon atoms.
Many candidates could explain the importance of chirality in drug action with an example.
The use of chiral auxiliaries was less known, many candidates failed to recognize that they are chiral themselves and many confusing answers were given.

