| Date | November 2009 | Marks available | 1 | Reference code | 09N.1.hl.TZ0.24 |
| Level | HL | Paper | 1 | Time zone | TZ0 |
| Command term | Determine | Question number | 24 | Adapted from | N/A |
Question
According to the Brønsted-Lowry theory, how does each species act in the equilibrium below?
\[{\text{C}}{{\text{H}}_3}{\text{COOH}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{COOH}}_2^ + + {\text{HSO}}_4^ - \]
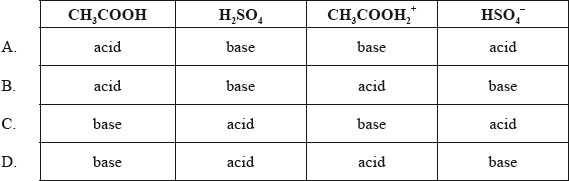
Markscheme
D
Examiners report
[N/A]
Syllabus sections
Show 53 related questions
- 17N.2.sl.TZ0.5c: A student working in the laboratory classified HNO3, H2SO4, H3PO4 and HClO4 as acids based on...
- 17N.2.sl.TZ0.5b.iii: State the conjugate base of the hydroxide ion, OH–.
- 17N.2.sl.TZ0.5b.ii: State what is meant by the term conjugate base.
- 17N.2.sl.TZ0.5b.i: Identify two different amphiprotic species in the above reactions.
- 17M.2.sl.TZ2.7b.i: Calculate the amount, in mol, of NaOH(aq) used.
- 17M.2.sl.TZ2.7a.ii: Identify one conjugate acid-base pair in the reaction.
- 17M.2.sl.TZ2.7a.i: Identify the amphiprotic species.
- 17M.1.sl.TZ2.20: Which of the following is correct? A. A weak acid is a proton donor and its aqueous...
- 17M.1.hl.TZ1.26: Which species acts as a Lewis and Brønsted–Lowry base? A. [Al(H2O)6]3+ B. BF3 C....
- 17M.1.hl.TZ1.24: Which species produced by the successive dissociations of phosphoric acid, H3PO4,...
- 17M.1.sl.TZ1.19: Which is an acid-base conjugate pair? A. H3O+ / OH– B. H2SO4 / SO42– C. ...
- 16N.1.sl.TZ0.19: Which species behave as Brønsted–Lowry bases in the following reaction? H2SO4 + HNO3...
- 16M.2.hl.TZ0.1b: Phosphine is usually prepared by heating white phosphorus, one of the allotropes of...
- 16M.2.hl.TZ0.1a: (i) Draw a Lewis (electron dot) structure of phosphine. (ii) State the hybridization of the...
- 16M.2.sl.TZ0.1b: Phosphine is usually prepared by heating white phosphorus, one of the allotropes of...
- 16M.1.sl.TZ0.19: Which is a...
- 15M.2.hl.TZ1.2c: Predict and explain the pH of the following aqueous solutions, using equations to support...
- 15M.2.hl.TZ1.8d.i: State the formula of the conjugate base of chloroethanoic acid,...
- 15M.2.hl.TZ2.7e.ii: Define the term weak base according to the Brønsted–Lowry theory.
- 15M.2.sl.TZ1.6e: Explain whether BF3 can act as a Brønsted-Lowry acid, a Lewis acid or both.
- 15M.2.sl.TZ2.5f.ii: Define the term weak base according to the Brønsted-Lowry theory.
- 15M.2.sl.TZ2.5f.iii: Deduce the formulas of conjugate acid-base pairs in the reaction...
- 14M.1.hl.TZ2.26: What is the conjugate base of phenol,...
- 14M.1.sl.TZ1.21: Which are acid-base pairs according to the Brønsted‒Lowry theory? I. ...
- 14N.2.hl.TZ0.9g: Describe what is meant by a weak Brønsted-Lowry base, including an equation for the reaction...
- 14N.2.sl.TZ0.6d.iv: State the formula of the conjugate base of...
- 13N.1.hl.TZ0.25: What are the conjugate acid–base pairs in the following...
- 13N.2.hl.TZ0.7b.ii: Explain the behaviour of HF in terms of the Brønsted–Lowry theory of acids.
- 13N.1.sl.TZ0.21: What are the conjugate acid–base pairs in the following...
- 13N.2.sl.TZ0.5c.i: Define a Brønsted–Lowry acid.
- 13M.1.hl.TZ2.26: Which of the following is an example of a Lewis acid–base reaction, but not a Brønsted–Lowry...
- 13M.2.hl.TZ2.6b.i: Define an acid according to the Brønsted–Lowry theory and the Lewis...
- 12N.1.sl.TZ0.22: Which row correctly describes...
- 12N.2.sl.TZ0.5b.i: Define an acid according to the Brønsted–Lowry and Lewis theories. Brønsted–Lowry...
- 10N.2.hl.TZ0.7d: The reaction between \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(aq)}}\) and...
- 10N.1.sl.TZ0.21: What is the conjugate base of \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\)...
- 09N.1.sl.TZ0.22: What is the formula of the conjugate base of the hydrogenphosphate ion,...
- 09N.2.sl.TZ0.6a.i: For each of the reactions A and B, deduce whether water is acting as an acid or a base and...
- 10M.2.sl.TZ1.3b.iii: State an equation, including state symbols, for the reaction of ethanoic acid with water....
- 10M.1.sl.TZ2.22: Which species behave as Brønsted-Lowry acids in the following reversible...
- 09M.2.sl.TZ1.6c.i: Define a Brønsted-Lowry acid.
- 09M.2.sl.TZ1.6c.ii: Deduce the two acids and their conjugate bases in the following...
- 09M.1.sl.TZ2.21: Which are definitions of an acid according to the Brønsted-Lowry and Lewis theories?
- 09M.2.sl.TZ2.1b.vii: The other product of the reaction is ethanoic acid,...
- 11M.2.hl.TZ1.8a.i: Define the terms acid and base according to the Brønsted-Lowry theory. Distinguish between a...
- 11M.1.sl.TZ1.22: Consider the equilibrium...
- 11M.2.sl.TZ1.5c.i: Define the terms acid and base according to the Brønsted-Lowry theory and state one example...
- 11M.1.sl.TZ2.20: Which is not a conjugate acid-base pair? A. \({\text{HN}}{{\text{O}}_{\text{3}}}\) and...
- 11M.2.sl.TZ2.5a.v: Explain, using the Brønsted-Lowry theory, how water can act either as an acid or a base. In...
- 12M.1.sl.TZ2.21: What is the Brønsted–Lowry conjugate base of \({{\text{H}}_{\text{2}}}{\text{PO}}_4^ -...
- 12M.2.sl.TZ2.6b.i: State the equation for the reaction of ammonia with water.
- 12M.2.sl.TZ2.6b.ii: Explain why ammonia can act as a Brønsted–Lowry base.
- 11N.1.sl.TZ0.21: Which descriptions are correct for both a Brønsted–Lowry acid and a Lewis acid?

