| Date | May 2014 | Marks available | 7 | Reference code | 14M.2.sl.TZ1.6 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Draw | Question number | 6 | Adapted from | N/A |
Question
Oxidation and reduction can be defined in terms of electron transfer or oxidation numbers.
Alcohols with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) occur as four structural isomers. Three of the isomers can be oxidized with acidified potassium dichromate solution to form compounds with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). The half-equation for the dichromate ion is:
\[{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {\text{14}}{{\text{H}}^ + }{\text{(aq)}} + {\text{6}}{{\text{e}}^ - } \rightleftharpoons {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}\]
Electrolysis has made it possible to obtain reactive metals from their ores.
A reactivity series can be experimentally determined by adding the metals W, X, Y and Z to solutions of these metal ions. The following reactions were observed:
\({{\text{W}}^{2 + }}{\text{(aq)}} + {\text{X(s)}} \to {\text{W(s)}} + {{\text{X}}^{2 + }}{\text{(aq)}}\)
\({\text{Y(s)}} + {{\text{W}}^{2 + }}{\text{(aq)}} \to {{\text{Y}}^{2 + }}{\text{(aq)}} + {\text{W(s)}}\)
\({{\text{Z}}^{2 + }}{\text{(aq)}} + {\text{W(s)}} \to {\text{Z(s)}} + {{\text{W}}^{2 + }}{\text{(aq)}}\)
\({\text{Y(s)}} + {{\text{X}}^{2 + }}{\text{(aq)}} \to {{\text{Y}}^{2 + }}{\text{(aq)}} + {\text{X(s)}}\)
Define oxidation in terms of electron transfer.
(i) Deduce the oxidation number of chromium in \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{2 - }\).
(ii) Deduce the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\).
(iii) Deduce the overall equation for the redox reaction.
(iv) Two of the isomers with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) can be oxidized further to form compounds with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\). Deduce the structural formulas of these two isomers.
(v) One isomer cannot be oxidized by acidified potassium dichromate solution.
Deduce its structural formula, state its name and identify it as a primary, secondary or tertiary alcohol.
Name:
Alcohol:
(vi) All isomers of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) undergo complete combustion. State an equation for the complete combustion of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\).
(i) Draw a labelled electrolytic cell for the electrolysis of molten potassium bromide, KBr. Include the direction of electron flow, the positive electrode (anode) and the negative electrode (cathode), the location of oxidation and reduction, and the electrolyte.
(ii) Deduce a half-equation for the reaction that occurs at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
(iii) Describe how current is conducted in a molten electrolyte.
(i) Deduce the order of reactivity of these four metals, from the least to the most reactive.
(ii) A voltaic cell is made by connecting a half-cell of X in \({\text{XC}}{{\text{l}}_{\text{2}}}{\text{(aq)}}\) to a half-cell of Z in \({\text{ZC}}{{\text{l}}_{\text{2}}}{\text{(aq)}}\). Deduce the overall equation for the reaction taking place when the cell is operating.
Markscheme
loss of electrons;
(i) \( + 6{\text{/VI}}\);
Do not award mark if incorrect notation used, ie, 6 , 6+ or –6.
(ii) \({{\text{C}}_4}{{\text{H}}_9}{\text{OH(l)}} \to {{\text{C}}_4}{{\text{H}}_8}{\text{O(l)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\);
Ignore state symbols.
(iii) \({\text{3}}{{\text{C}}_4}{{\text{H}}_9}{\text{OH(l)}} + {\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{3}}{{\text{C}}_4}{{\text{H}}_8}{\text{O(l)}} + {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}\);
Ignore state symbols.
(iv) \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{OH}}\);
\({{\text{(C}}{{\text{H}}_3}{\text{)}}_2}{\text{CHC}}{{\text{H}}_2}{\text{OH}}\);
Accept full or condensed structural formulas.
(v) \({{\text{(C}}{{\text{H}}_3}{\text{)}}_3}{\text{COH}}\);
2-methylpropan-2-ol;
Allow 2-methyl-2-propanol , methylpropan-2-ol, methyl-2-propanol.
tertiary;
(vi) \({{\text{C}}_4}{{\text{H}}_9}{\text{OH}} + {\text{6}}{{\text{O}}_2} \to {\text{4C}}{{\text{O}}_2} + {\text{5}}{{\text{H}}_2}{\text{O}}/{{\text{(C}}{{\text{H}}_3}{\text{)}}_3}{\text{COH}} + {\text{6}}{{\text{O}}_2} \to {\text{4C}}{{\text{O}}_2} + {\text{5}}{{\text{H}}_2}{\text{O}}\)
correct reactants and products;
correct balancing;
(i) (DC) power supply
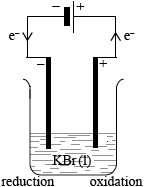
(DC) power supply / battery;
electrodes labelled as +/anode or –/cathode and electron flow;
reduction at negative electrode (cathode) / oxidation at positive electrode (anode);
electrolyte / molten KBr/KBr(l) / \({{\text{K}}^ + }{\text{(l)}}\) and \({\text{B}}{{\text{r}}^ - }{\text{(l)}}\);
(ii) Positive electrode (anode):
\({\text{2B}}{{\text{r}}^ - }{\text{(l)}} \to {\text{B}}{{\text{r}}_2}{\text{(l)}} + {\text{2}}{{\text{e}}^ - }\);
Negative electrode (cathode):
\({{\text{K}}^ + }{\text{(l)}} + {{\text{e}}^ - } \to {\text{K(l)}}\);
Award [1 max] if correct half-equations are given at the wrong electrodes.
Allow e instead of e–.
Ignore state symbols.
Penalize equilibrium sign once only.
(iii) positive ions move towards negative electrode (cathode) and negative ions move towards positive electrode (anode) / ions move to oppositely charged electrode / negative ions give up electrons at positive electrode and positive ions gain electrons at negative electrode;
(i) \({\text{Z}} < {\text{W}} < {\text{X}} < {\text{Y}}\);
Accept \(Y > X > W > Z\).
(ii) \({\text{X(s)}} + {{\text{Z}}^{2 + }}{\text{(aq)}} \to {{\text{X}}^{2 + }}{\text{(aq)}} + {\text{Z(s)}}\);
Ignore state symbols.
Accept X(s) + ZCl2(aq) \( \to \) XCl2(aq) + Z(s).
Examiners report
This was another popular choice of question in Section B. In part (a) almost all candidates defined oxidation correctly. The oxidation number of chromium was mostly determined correctly in (b)(i), but only the better candidates could write the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) in (b)(ii), even though the product was identified in the question as \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). Subsequently the overall equation of the redox reaction in (b)(iii) was poorly answered. One respondent stated that balanced redox equations are not required. This is stated in 9.2.2. In (b)(iv), candidates who realized the product with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\) was an acid, deduced correct formulas of the two primary alcohols, though some did not read the question and gave the formulae for the acid and not the alcohol. Commonly, candidates drew isomers of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) giving one primary structure and one secondary structure. Incorrect structures frequently had oxygen atoms connected to the molecule with single bonds but nothing else attached. Part (v) required candidates to identify the isomer which cannot be oxidized by acidified potassium dichromate solution. Many candidates correctly gave the formula and name of the tertiary alcohol. In (b)(vi) several candidates gave a correct equation for the combustion of alcohols but more usually one mark was scored for correct reactants and products and the mark for correct balancing was missed. Part (c) was on electrolysis. There were several poorly drawn electrolytic cells in (c)(i), sometimes even with the electrodes outside of the electrolyte, but most candidates managed a few marks and many candidates scored full marks. A significant number of candidates drew a voltaic cell with a salt bridge and a small minority had the battery terminals incorrectly connected or drew a voltmeter. The half-equations for electrode reactions were poorly done in (c)(ii) with several candidates again writing whole equations. The reduction of the potassium ion was often given at the anode and the oxidation of the bromide ion was seldom done well with many candidates writing a reduction half-equation for Br2. In (c)(iii) candidates described poorly how current is conducted in a molten electrolyte. The common response was that electrons are forced through the solution from the cathode to the anode. Many candidates deduced a correct order of reactivity for the metals listed in (d)(i) but the overall equation for the reaction occurring in a voltaic cell made from two of the metals was either done well or was completely wrong in (d)(ii).
This was another popular choice of question in Section B. In part (a) almost all candidates defined oxidation correctly. The oxidation number of chromium was mostly determined correctly in (b)(i), but only the better candidates could write the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) in (b)(ii), even though the product was identified in the question as \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). Subsequently the overall equation of the redox reaction in (b)(iii) was poorly answered. One respondent stated that balanced redox equations are not required. This is stated in 9.2.2. In (b)(iv), candidates who realized the product with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\) was an acid, deduced correct formulas of the two primary alcohols, though some did not read the question and gave the formulae for the acid and not the alcohol. Commonly, candidates drew isomers of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) giving one primary structure and one secondary structure. Incorrect structures frequently had oxygen atoms connected to the molecule with single bonds but nothing else attached. Part (v) required candidates to identify the isomer which cannot be oxidized by acidified potassium dichromate solution. Many candidates correctly gave the formula and name of the tertiary alcohol. In (b)(vi) several candidates gave a correct equation for the combustion of alcohols but more usually one mark was scored for correct reactants and products and the mark for correct balancing was missed. Part (c) was on electrolysis. There were several poorly drawn electrolytic cells in (c)(i), sometimes even with the electrodes outside of the electrolyte, but most candidates managed a few marks and many candidates scored full marks. A significant number of candidates drew a voltaic cell with a salt bridge and a small minority had the battery terminals incorrectly connected or drew a voltmeter. The half-equations for electrode reactions were poorly done in (c)(ii) with several candidates again writing whole equations. The reduction of the potassium ion was often given at the anode and the oxidation of the bromide ion was seldom done well with many candidates writing a reduction half-equation for Br2. In (c)(iii) candidates described poorly how current is conducted in a molten electrolyte. The common response was that electrons are forced through the solution from the cathode to the anode. Many candidates deduced a correct order of reactivity for the metals listed in (d)(i) but the overall equation for the reaction occurring in a voltaic cell made from two of the metals was either done well or was completely wrong in (d)(ii).
This was another popular choice of question in Section B. In part (a) almost all candidates defined oxidation correctly. The oxidation number of chromium was mostly determined correctly in (b)(i), but only the better candidates could write the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) in (b)(ii), even though the product was identified in the question as \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). Subsequently the overall equation of the redox reaction in (b)(iii) was poorly answered. One respondent stated that balanced redox equations are not required. This is stated in 9.2.2. In (b)(iv), candidates who realized the product with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\) was an acid, deduced correct formulas of the two primary alcohols, though some did not read the question and gave the formulae for the acid and not the alcohol. Commonly, candidates drew isomers of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) giving one primary structure and one secondary structure. Incorrect structures frequently had oxygen atoms connected to the molecule with single bonds but nothing else attached. Part (v) required candidates to identify the isomer which cannot be oxidized by acidified potassium dichromate solution. Many candidates correctly gave the formula and name of the tertiary alcohol. In (b)(vi) several candidates gave a correct equation for the combustion of alcohols but more usually one mark was scored for correct reactants and products and the mark for correct balancing was missed. Part (c) was on electrolysis. There were several poorly drawn electrolytic cells in (c)(i), sometimes even with the electrodes outside of the electrolyte, but most candidates managed a few marks and many candidates scored full marks. A significant number of candidates drew a voltaic cell with a salt bridge and a small minority had the battery terminals incorrectly connected or drew a voltmeter. The half-equations for electrode reactions were poorly done in (c)(ii) with several candidates again writing whole equations. The reduction of the potassium ion was often given at the anode and the oxidation of the bromide ion was seldom done well with many candidates writing a reduction half-equation for Br2. In (c)(iii) candidates described poorly how current is conducted in a molten electrolyte. The common response was that electrons are forced through the solution from the cathode to the anode. Many candidates deduced a correct order of reactivity for the metals listed in (d)(i) but the overall equation for the reaction occurring in a voltaic cell made from two of the metals was either done well or was completely wrong in (d)(ii).
This was another popular choice of question in Section B. In part (a) almost all candidates defined oxidation correctly. The oxidation number of chromium was mostly determined correctly in (b)(i), but only the better candidates could write the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) in (b)(ii), even though the product was identified in the question as \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). Subsequently the overall equation of the redox reaction in (b)(iii) was poorly answered. One respondent stated that balanced redox equations are not required. This is stated in 9.2.2. In (b)(iv), candidates who realized the product with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\) was an acid, deduced correct formulas of the two primary alcohols, though some did not read the question and gave the formulae for the acid and not the alcohol. Commonly, candidates drew isomers of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) giving one primary structure and one secondary structure. Incorrect structures frequently had oxygen atoms connected to the molecule with single bonds but nothing else attached. Part (v) required candidates to identify the isomer which cannot be oxidized by acidified potassium dichromate solution. Many candidates correctly gave the formula and name of the tertiary alcohol. In (b)(vi) several candidates gave a correct equation for the combustion of alcohols but more usually one mark was scored for correct reactants and products and the mark for correct balancing was missed. Part (c) was on electrolysis. There were several poorly drawn electrolytic cells in (c)(i), sometimes even with the electrodes outside of the electrolyte, but most candidates managed a few marks and many candidates scored full marks. A significant number of candidates drew a voltaic cell with a salt bridge and a small minority had the battery terminals incorrectly connected or drew a voltmeter. The half-equations for electrode reactions were poorly done in (c)(ii) with several candidates again writing whole equations. The reduction of the potassium ion was often given at the anode and the oxidation of the bromide ion was seldom done well with many candidates writing a reduction half-equation for Br2. In (c)(iii) candidates described poorly how current is conducted in a molten electrolyte. The common response was that electrons are forced through the solution from the cathode to the anode. Many candidates deduced a correct order of reactivity for the metals listed in (d)(i) but the overall equation for the reaction occurring in a voltaic cell made from two of the metals was either done well or was completely wrong in (d)(ii).

