| Date | May 2015 | Marks available | 2 | Reference code | 15M.2.sl.TZ2.3 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Describe | Question number | 3 | Adapted from | N/A |
Question
Electrolysis is an important industrial process used to obtain very reactive elements from their common ores.
Molten magnesium chloride can be electrolysed using inert graphite electrodes at 800 °C.
Describe, using a labelled diagram, the essential components of this electrolytic cell.
Molten magnesium chloride can be electrolysed using inert graphite electrodes at 800 °C.
Deduce the half-equations, including state symbols, for the reactions occurring at each electrode. (The melting points of MgCl2 and Mg are 714 °C and 649 °C respectively.)
Positive electrode (anode):
Negative electrode (cathode):
Outline why solid magnesium chloride does not conduct electricity.
Aluminium can also be obtained by electrolysis. Suggest one reason why aluminium is often used instead of iron by engineers.
Markscheme
Cell showing:
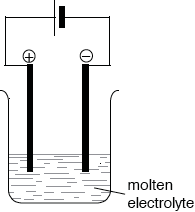
molten electrolyte/MgCl2(l), electrodes and battery/DC supply;
correct labelling of positive electrode/anode/+ and negative electrode/cathode/–;
Positive electrode (anode):
\(2{\text{C}}{{\text{l}}^ - }{\text{(l)}} \to {\text{C}}{{\text{l}}_2}{\text{(g)}} + 2{{\text{e}}^ - }/{\text{C}}{{\text{l}}^ - }{\text{(l)}} \to \frac{1}{2}{\text{C}}{{\text{l}}_2}{\text{(g)}} + {{\text{e}}^ - }\);
Negative electrode (cathode):
\({\text{M}}{{\text{g}}^{2 + }}{\text{(l)}} + 2{{\text{e}}^ - } \to {\text{Mg(l)}}\);
Accept e instead of e–.
Award [1 max] for correct half-equations given at the wrong electrode.
Penalize use of reversible arrows once only.
correct state symbols in both equations;
ions are not free to move when solid / ions in rigid lattice / OWTTE;
aluminium/Al is less dense (compared to iron/Fe) / Al is more ductile or malleable/ aluminium forms a protective oxide layer / Al does not corrode / iron/Fe rusts /OWTTE;
Do not accept “Al is lighter” OR “less expensive” OR “Al can be recycled”.
Examiners report
There were very few carefully drawn correct diagrams as well as too many diagrams showing half-cells. The importance of the solution being molten was not appreciated. The equations did pick up marks, but it was extremely rare for candidates to access the mark for the correct state symbols. Far too many associated electrical conductivity in molten compounds with mobile electrons. The awareness that mobile ions are responsible for conductivity was poorly understood. The difference between "lightness" and density is still confused.
There were very few carefully drawn correct diagrams as well as too many diagrams showing half-cells. The importance of the solution being molten was not appreciated. The equations did pick up marks, but it was extremely rare for candidates to access the mark for the correct state symbols. Far too many associated electrical conductivity in molten compounds with mobile electrons. The awareness that mobile ions are responsible for conductivity was poorly understood. The difference between "lightness" and density is still confused.
There were very few carefully drawn correct diagrams as well as too many diagrams showing half-cells. The importance of the solution being molten was not appreciated. The equations did pick up marks, but it was extremely rare for candidates to access the mark for the correct state symbols. Far too many associated electrical conductivity in molten compounds with mobile electrons. The awareness that mobile ions are responsible for conductivity was poorly understood. The difference between "lightness" and density is still confused.
There were very few carefully drawn correct diagrams as well as too many diagrams showing half-cells. The importance of the solution being molten was not appreciated. The equations did pick up marks, but it was extremely rare for candidates to access the mark for the correct state symbols. Far too many associated electrical conductivity in molten compounds with mobile electrons. The awareness that mobile ions are responsible for conductivity was poorly understood. The difference between "lightness" and density is still confused.

