| Date | May 2015 | Marks available | 1 | Reference code | 15M.1.sl.TZ2.25 |
| Level | SL | Paper | 1 | Time zone | TZ2 |
| Command term | Question number | 25 | Adapted from | N/A |
Question
A voltaic cell is made by connecting a copper half-cell, \({\text{Cu(s)}}\left| {{\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}} \right.\), to an iron half-cell \({\text{Fe(s)}}\left| {{\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}} \right.\).
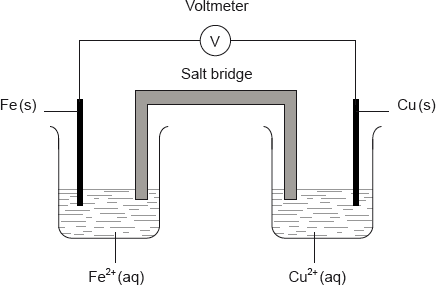
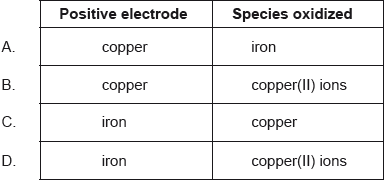
Which combination correctly identifies the positive electrode and the species being oxidized?
Markscheme
A
Examiners report
Questions such as this have been set in the past and we would expect a chemist at this level to have a rudimentary knowledge of metals in an activity series, particularly those as far apart as iron and copper. It was disappointing that less than 50% got this right.
Syllabus sections
Show 72 related questions
- 17N.3.sl.TZ0.1b.iii: Outline how current flows in the sodium chloride solution.
- 17N.3.sl.TZ0.1a: Sketch a graph that would support the student’s hypothesis.
- 17N.2.sl.TZ0.2e.iii: The voltaic cell stated in part (ii) is partially shown below. Draw and label the...
- 17N.2.sl.TZ0.2e.ii: A voltaic cell is made up of a Mn2+/Mn half-cell and a Ni2+/Ni half-cell. Deduce the...
- 17N.1.sl.TZ0.23: What is the reaction type and major product at the anode (positive electrode) when molten...
- 17N.2.hl.TZ0.7b: Predict, giving a reason, the direction of movement of electrons when the standard nickel and...
- 17N.2.hl.TZ0.7a: Deduce a balanced equation for the overall reaction when the standard nickel and iodine...
- 17M.1.sl.TZ2.23: What occurs at the anode (positive electrode) during the electrolysis of molten strontium...
- 17M.1.sl.TZ1.23: Which statements are correct for a voltaic...
- 16N.2.hl.TZ0.4i: Magnesium chloride can be electrolysed. (i) Deduce the half-equations for the reactions at...
- 16N.2.sl.TZ0.4g: Magnesium chloride can be electrolysed. Deduce the half-equations for the reactions at each...
- 16N.1.sl.TZ0.22: A voltaic cell is constructed from zinc and copper half-cells. Zinc is more reactive than...
- 16M.1.hl.TZ0.31: Which...
- 16M.1.sl.TZ0.22: Which statement is correct for a voltaic but not for an electrolytic cell? A. An...
- 15M.2.hl.TZ1.6d.i: Bromine can be produced by the electrolysis of molten sodium bromide. Deduce the...
- 15M.1.sl.TZ1.25: Two half-cells are connected via a salt bridge to make a voltaic cell. Which statement about...
- 15M.2.sl.TZ1.2c: Bromine can be produced by the electrolysis of molten sodium bromide. Deduce the...
- 15M.2.sl.TZ2.3a.i: Describe, using a labelled diagram, the essential components of this electrolytic cell.
- 15M.2.sl.TZ2.3a.ii: Molten magnesium chloride can be electrolysed using inert graphite electrodes at 800...
- 14M.2.hl.TZ1.6b: (i) Deduce the order of reactivity of these four metals, from the least to the most...
- 14M.2.hl.TZ2.8g: (i) Draw a labelled diagram of a suitable apparatus for the electrolysis. (ii) ...
- 14M.1.sl.TZ1.25: At which electrodes does oxidation occur in a voltaic cell and in an electrolytic cell?
- 14M.1.sl.TZ2.25: Which process occurs when a molten salt is electrolysed? A. The metal ion is oxidized...
- 14M.1.sl.TZ2.24: Zinc is more reactive than copper. In this voltaic cell, which species is reduced and in...
- 14M.2.sl.TZ1.6d: (i) Deduce the order of reactivity of these four metals, from the least to the most...
- 14M.2.sl.TZ1.6c: (i) Draw a labelled electrolytic cell for the electrolysis of molten potassium bromide,...
- 14N.1.hl.TZ0.31: Which species are produced at each electrode during the electrolysis of molten lead(II)...
- 14N.1.sl.TZ0.25: Which statement about an electrolytic cell is correct? A. Chemical energy is converted...
- 14N.2.sl.TZ0.8c: (i) Given that magnesium is more reactive than silver, deduce the half-equations for the...
- 14N.3.sl.TZ0.8a: Deduce an equation for the discharge of the ions at each electrode. Positive electrode...
- 13N.1.sl.TZ0.25: Which statements are correct for the electrolysis of molten lead(II) bromide,...
- 13N.2.sl.TZ0.5b.iv: A voltaic cell can be constructed using cadmium and europium half-cells. State how the two...
- 13M.1.hl.TZ1.32: Which are correct statements about a voltaic cell? I. A spontaneous redox reaction...
- 13M.2.hl.TZ1.7c.i: Describe, using a diagram, the essential components of an electrolytic cell.
- 13M.2.hl.TZ1.7c.ii: Describe the two ways in which current is conducted in an electrolytic cell.
- 13M.2.sl.TZ1.3b: Sodium can be obtained by electrolysis from molten sodium chloride. Describe, using a...
- 13M.2.hl.TZ2.7e.vi: Draw a fully labelled diagram of the electrolytic cell, showing the positive electrode...
- 13M.2.hl.TZ2.7e.v: Deduce the overall cell reaction, including state symbols.
- 12N.1.sl.TZ0.24: A voltaic cell is made by connecting zinc and lead half-cells. The overall equation for the...
- 12N.1.sl.TZ0.25: Which process occurs during the electrolysis of molten sodium chloride? A. Oxidation...
- 10N.2.hl.TZ0.4b: (i) Explain how molten magnesium chloride conducts an electric current. (ii) ...
- 10N.1.sl.TZ0.25: Which statement is correct for the electrolysis of molten lead iodide,...
- 10N.1.sl.TZ0.24: Metal A is more reactive than metal B. A standard voltaic cell is made as shown. Which...
- 09N.1.hl.TZ0.30: In the electrolytic cell shown, at which electrode will chlorine form, and what is the...
- 09N.2.hl.TZ0.4b.iii: On the cell diagram below, label the negative electrode (anode), the positive electrode...
- 09N.2.hl.TZ0.4a: Outline two differences between an electrolytic cell and a voltaic cell.
- 10M.2.sl.TZ1.5c: (i) Solid sodium chloride does not conduct electricity but molten sodium chloride does....
- 10M.2.sl.TZ1.5a: (i) Draw an annotated diagram of a voltaic cell composed of a magnesium electrode in...
- 10M.1.sl.TZ2.26: Which changes could take place at the positive electrode (cathode) in a voltaic cell? I. ...
- 10M.2.sl.TZ2.5c: (i) Determine the oxidation number of lead in Pb,...
- 09M.2.hl.TZ1.7d.i: concentrated sodium chloride
- 09M.2.hl.TZ1.7d.ii: molten sodium bromide
- 09M.1.sl.TZ1.25: Consider how current is conducted in an electrolytic cell. Which statement is correct? A. ...
- 09M.2.sl.TZ1.3b: Molten sodium oxide is a good conductor of electricity. State the half-equation for the...
- 09M.1.sl.TZ1.24: What happens at the negative electrode in a voltaic cell and in an electrolytic cell?
- 09M.2.hl.TZ2.6c.i: Outline two differences between an electrolytic cell and a voltaic cell.
- 09M.2.sl.TZ2.5b.iv: Molten sodium chloride undergoes electrolysis in an electrolytic cell. For each electrode...
- 09M.2.sl.TZ2.5b.ii: Describe using a labelled diagram, the essential components of an electrolytic cell.
- 09M.2.sl.TZ2.5b.v: Electrolysis has made it possible to obtain reactive metals such as aluminium from their...
- 09M.2.sl.TZ2.5b.vi: Outline two differences between an electrolytic cell and a voltaic cell.
- 11M.2.hl.TZ1.9a.i: Draw an electrolytic cell illustrating the electrolysis of molten nickel(II) bromide,...
- 11M.1.sl.TZ1.24: Consider the overall reaction taking place in a voltaic...
- 11M.2.hl.TZ2.7c.i: Draw a labelled diagram of a voltaic cell made from an Fe (s) /...
- 11M.2.hl.TZ2.7c.ii: Deduce the equation for the chemical reaction occurring when the cell in part (c) (i) is...
- 11M.2.hl.TZ2.7e.i: Explain why it is very difficult to obtain sodium from sodium chloride by any other method.
- 11M.1.sl.TZ2.25: Which statement about the electrolysis of molten sodium chloride is correct? A. A...
- 11M.2.sl.TZ2.5b.i: Draw a labelled diagram of a voltaic cell made from an...
- 11M.2.sl.TZ2.5b.ii: Deduce the half-equations for the reactions taking place at the positive electrode (cathode)...
- 12M.1.sl.TZ2.25: What occurs during the operation of a voltaic cell based on the following overall...
- 11N.2.sl.TZ0.3a.i: Draw the essential components of this electrolytic cell and identify the products that form...
- 11N.2.sl.TZ0.3a.ii: State the half-equations for the oxidation and reduction processes and deduce the overall...
- 11N.1.sl.TZ0.24: What is produced at the positive electrode (anode) and negative electrode (cathode) during...

