| Date | May 2014 | Marks available | 1 | Reference code | 14M.3.hl.TZ2.4 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Suggest | Question number | 4 | Adapted from | N/A |
Question
Methylene blue can be used as an indicator.
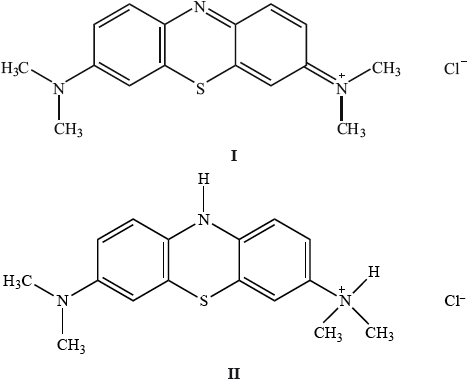
Explain which of the two structures would be coloured.
In terms of the wavelength of the visible light absorbed, suggest why the coloured form is blue.
Markscheme
I;
more conjugation/delocalization of electrons / more alternating C–C and C=C
and
the less energy required to excite electrons / absorbs in visible region/at longer wavelength/lower frequency;
absorbs red/orange/yellow/long wavelength visible light (hence appears as the complementary colour);
Examiners report
Candidates had little difficulty in choosing compound I, knew about conjugation but tended to omit the absorption of light in the visible region (or equivalent). Although it wasn’t penalized at this point, there are still candidates talking about reflected light. Students should have some knowledge of complementary colours without having specifically memorized the colour wheel.
Candidates had little difficulty in choosing compound I, knew about conjugation but tended to omit the absorption of light in the visible region (or equivalent). Although it wasn’t penalized at this point, there are still candidates talking about reflected light. Students should have some knowledge of complementary colours without having specifically memorized the colour wheel.

