| Date | May 2012 | Marks available | 2 | Reference code | 12M.3.hl.TZ2.D3 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Describe | Question number | D3 | Adapted from | N/A |
Question
The discovery of penicillin by Alexander Fleming in 1928 is often given as an example of serendipity in science.
The structure of a particular type of penicillin called dicloxacillin is shown below.
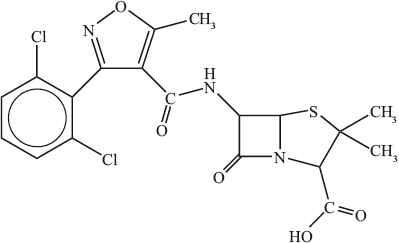
Describe what happens to bacteria when they come into contact with penicillin.
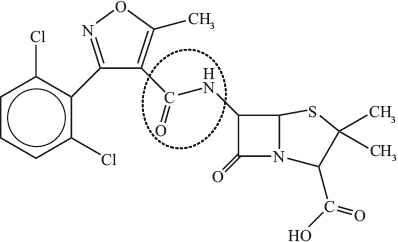
(i) Identify the \(\beta \)-lactam ring by drawing a circle around it.
(ii) Explain why the \(\beta \)-lactam ring is so important in the mechanism of the action
of penicillin.
(iii) State the name of the functional group in dicloxacillin, circled below.
Comment on the fact that many bacteria are now resistant to penicillins.
Markscheme
interferes with enzymes/chemicals that bacteria need to make cell walls / interferes with cell wall formation;
osmosis/osmotic pressure causes cell wall to break/burst / water enters cell causing it to burst / OWTTE;
(i) 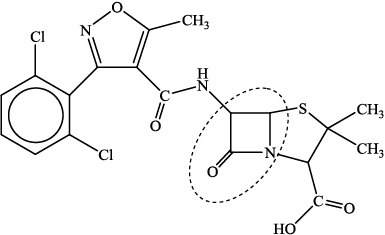 ;
;
(ii) ring strain / bond angles are approx 90° / should be 109° or 120° / OWTTE;
ring breaks / produces reactive amide group / OWTTE;
(so) penicillin can become bonded to enzyme/penicillinase;
(iii) amide;
caused by overprescription/overuse/overdose / not completing course of penicillin / use of antibiotics in animal feed / OWTTE;
penicillins with modified side chains must be developed/cocktail of drugs must be taken to overcome resistant bacteria / OWTTE;
Examiners report
Most answers scored at least one mark in (c), or came close to it – most that did not were not specific enough or failed to mention cell walls.
In (d), the \(\beta \)-lactam ring was usually correctly identified, as was the circled amide group. In part (d)(ii), few answers scored full marks, although most identified the relevance of the bond angles in causing ring strain.
In (e), many more scored the overprescription mark than the one for modifying the side chain.

