| Date | May 2018 | Marks available | 1 | Reference code | 18M.1.HL.TZ1.38 |
| Level | Higher level | Paper | Paper 1 | Time zone | 1 |
| Command term | Question number | 38 | Adapted from | N/A |
Question
According to the Bohr model for hydrogen, visible light is emitted when electrons make transitions from excited states down to the state with n = 2. The dotted line in the following diagram represents the transition from n = 3 to n = 2 in the spectrum of hydrogen.
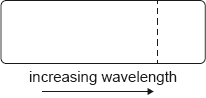
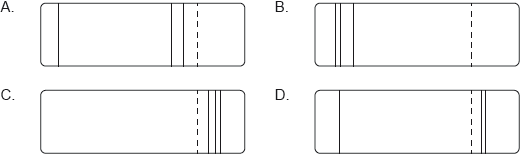
Which of the following diagrams could represent the visible light emission spectrum of hydrogen?
Markscheme
B
Examiners report
Syllabus sections
-
22M.1.HL.TZ2.39:
The dashed line represents the variation with incident electromagnetic frequency of the kinetic energy EK of the photoelectrons ejected from a metal surface. The metal surface is then replaced with one that requires less energy to remove an electron from the surface.
Which graph of the variation of EK with will be observed?
-
22M.1.HL.TZ2.40:
Which graph shows a possible probability density function for a given wave function of an electron?
- 22M.2.HL.TZ2.9a.i: Identify a property of electrons demonstrated by this experiment.
-
18M.2.HL.TZ1.8b.ii:
Suggest, with reference to conservation of energy, how the variable voltage source can be used to stop all emitted electrons from reaching the collecting plate.
- 17N.1.HL.TZ0.39: Monochromatic electromagnetic radiation is incident on a metal surface. The kinetic energy of...
- 17M.2.HL.TZ1.9b.iii: Calculate the work function of barium in eV.
- 22M.1.HL.TZ2.37: Three correct statements about the behaviour of electrons are: I. An electron beam is used...
- 22M.1.HL.TZ1.39: What is evidence for wave–particle duality? A. Line spectra of elements B. ...
-
22M.1.HL.TZ1.38:
Light with photons of energy 8.0 × 10−20 J are incident on a metal surface in a photoelectric experiment.
The work function of the metal surface is 4.8 × 10−20 J . What minimum voltage is required for the ammeter reading to fall to zero?
A. 0.2 V
B. 0.3 V
C. 0.5 V
D. 0.8 V
- 16N.1.HL.TZ0.37: Pair production by a photon occurs in the presence of a nucleus. For this process, which of...
- 17M.1.HL.TZ1.39: A photon of energy E and wavelength λ is scattered from an electron initially at rest. What...
-
16N.2.HL.TZ0.11a:
A current is observed on the ammeter when violet light illuminates C. With V held constant the current becomes zero when the violet light is replaced by red light of the same intensity. Explain this observation.
- 18N.1.HL.TZ0.38: Which is the correct Feynman diagram for pair annihilation and pair production?
- 18N.1.HL.TZ0.37: When green light is incident on a clean zinc plate no photoelectrons are emitted. What...
- 19N.1.HL.TZ0.37: An electron of low energy is enclosed within a high potential barrier. What is the process by...
-
21N.1.HL.TZ0.38:
A beam of electrons moving in the direction shown is incident on a rectangular slit of width .
The component of momentum of the electrons in direction after passing through the slit is . The uncertainty in is
A. proportional toB. proportional to
C. proportional to
D. zero
- 19M.1.HL.TZ2.2: A proton has momentum 10-20 N s and the uncertainty in the position of the proton is 10-10 m....
- 19M.1.HL.TZ2.39: Three possible features of an atomic model are I. orbital radius II. quantized energy III....
-
19M.1.HL.TZ2.38:
Photons of a certain frequency incident on a metal surface cause the emission of electrons from the surface. The intensity of the light is constant and the frequency of photons is increased. What is the effect, if any, on the number of emitted electrons and the energy of emitted electrons?
-
17M.1.HL.TZ1.38:
What can be used to calculate the probability of finding an electron in a particular region of space?
A.
B.
C. The magnitude of the wave function
D. The magnitude of the (wave function)2
-
16N.2.HL.TZ0.11b:
The graph shows the variation of photoelectric current I with potential difference V between C and A when violet light of a particular intensity is used.
The intensity of the light source is increased without changing its wavelength.
(i) Draw, on the axes, a graph to show the variation of I with V for the increased intensity.
(ii) The wavelength of the violet light is 400 nm. Determine, in eV, the work function of caesium.
(iii) V is adjusted to +2.50V. Calculate the maximum kinetic energy of the photoelectrons just before they reach A.
- 19N.2.HL.TZ0.11a(i): does not support the wave nature of light.
- 19N.2.HL.TZ0.11a(ii): does support the photon nature of light.
-
17N.1.HL.TZ0.23:
Samples of different radioactive nuclides have equal numbers of nuclei. Which graph shows the relationship between the half-life and the activity A for the samples?
-
18M.2.HL.TZ2.9c.iii:
Calculate the electron’s orbital radius in (c)(ii).
- 17M.2.HL.TZ1.9b.ii: State what is meant by the work function of a metal.
- 17M.2.HL.TZ1.9c: The experiment is repeated with a metal surface of cadmium, which has a greater...
- 17M.2.HL.TZ2.7a.ii: Electrons emitted from the surface of the photocell have almost no kinetic energy. Explain...
- 19M.1.HL.TZ1.40: A particle is confined within a nucleus. What is the order of magnitude of the uncertainty in...
- 21N.2.HL.TZ0.4d.ii: State and explain what happens to the rate at which charge leaves the metallic surface.
- 17N.1.HL.TZ0.40: A photon interacts with a nearby nucleus to produce an electron. What is the name of this...
- 19M.1.HL.TZ1.38: A metallic surface is first irradiated with infrared radiation and photoelectrons are emitted...
-
18M.1.HL.TZ1.37:
Two radioactive nuclides, X and Y, have half-lives of 50 s and 100 s respectively. At time t = 0 samples of X and Y contain the same number of nuclei.
What is when t = 200 s?
A. 4
B. 2
C.
D.
- 19N.1.HL.TZ0.39: Three observations of the behaviour of electrons are I. electron emission as a result of...
-
19N.2.HL.TZ0.11b(ii):
The intensity of the light incident on the surface is reduced by half without changing the wavelength. Draw, on the graph, the variation of the current with potential after this change.
- 18M.1.HL.TZ2.38: Which of the following is evidence for the wave nature of the electron? A. Continuous...
-
19N.1.HL.TZ0.38:
A beam of monochromatic radiation is made up of photons each of momentum . The intensity of the beam is doubled without changing frequency. What is the momentum of each photon after the change?
A.
B.
C.
D.
-
17M.2.HL.TZ2.7c.ii:
State and explain the effect on the maximum photoelectric current as a result of increasing the photon energy in this way.
-
20N.2.HL.TZ0.10a:
Show that the wavelength of an electron in the beam is about .
-
16N.1.HL.TZ0.38:
An electron of mass m has an uncertainty in its position r. What is the uncertainty in the speed of this electron?
A.
B.
C.
D.
-
18M.2.HL.TZ1.8a:
Show that the energy of photons from the UV lamp is about 10 eV.
-
22M.2.HL.TZ2.9a.iii:
The de Broglie wavelength for an electron is given by . Show that the diameter of an oxygen-16 nucleus is about 4 fm.
-
19N.2.HL.TZ0.8b(i):
Show that the de Broglie wavelength of the electron in the state is m.
The formula for the de Broglie wavelength of a particle is .
- 20N.1.HL.TZ0.37: Monochromatic light is incident on a metal surface and electrons are released. The intensity...
-
20N.1.HL.TZ0.39:
A photon has a wavelength . What are the energy and momentum of the photon?
-
17M.1.HL.TZ2.39:
A neutron of mass m is confined within a nucleus of diameter d. Ignoring numerical constants, what is an approximate expression for the kinetic energy of the neutron?
A.
B.
C.
D.
-
19N.2.HL.TZ0.8b(ii):
Estimate for , the ratio .
State your answer to one significant figure.
- 21M.2.HL.TZ1.10a: Describe the photoelectric effect.
-
17M.1.HL.TZ2.38:
In the Bohr model for hydrogen an electron in the ground state has orbit radius r and speed v. In the first excited state the electron has orbit radius 4r. What is the speed of the electron in the first excited state?
A.
B.
C.
D.
- 20N.2.HL.TZ0.10b(i): Discuss how the results of the experiment provide evidence for matter waves.
- 21M.2.HL.TZ2.9a.i: Outline the cause of the electron emission for radiation A.
- 21M.2.HL.TZ2.9a.ii: Outline why electrons are never emitted for radiation C.
- 21M.2.HL.TZ2.9a.iii: Outline why radiation B gives different results.
- 21M.2.HL.TZ2.9b: Explain why there is no effect on the table of results when the intensity of source B is...
-
21M.1.HL.TZ1.38:
In a photoelectric effect experiment, a beam of light is incident on a metallic surface W in a vacuum.
The graph shows how the current varies with the potential difference V when three different beams X, Y, and Z are incident on W at different times.
I. X and Y have the same frequency.
II. Y and Z have different intensity.
III. Y and Z have the same frequency.Which statements are correct?
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
- 21M.1.HL.TZ1.37: What is a consequence of the uncertainty principle? A. The absorption spectrum of hydrogen...
-
21M.1.HL.TZ2.37:
A particle of energy is incident upon a barrier and has a certain probability of quantum tunnelling through the barrier. Assuming remains constant, which combination of changes in particle mass and barrier length will increase the probability of the particle tunnelling through the barrier?
- 21M.1.HL.TZ2.39: What is true for the Bohr model for the hydrogen atom? A. Angular momentum of electrons is...
-
19N.2.HL.TZ0.11b(i):
Calculate, in eV, the work function of the metal surface.
-
21M.2.HL.TZ1.10b:
Show that the maximum velocity of the photoelectrons is .
-
18M.2.HL.TZ1.8b.iii:
The variable voltage can be adjusted so that no electrons reach the collecting plate. Write down the minimum value of the voltage for which no electrons reach the collecting plate.
-
21M.1.HL.TZ2.40:
An electron of non-relativistic speed interacts with an atom. All the energy of the electron is transferred to an emitted photon of frequency . An electron of speed now interacts with the same atom and all its energy is transmitted to a second photon. What is the frequency of the second photon?
A.
B.
C.
D.
- 18M.1.HL.TZ2.37: A photoelectric cell is connected in series with a battery of emf 2 V. Photons of energy 6...
- 18M.2.HL.TZ1.8b.i: Calculate, in J, the maximum kinetic energy of the emitted electrons.
- 17M.2.HL.TZ2.7c.i: Describe the change in the number of photons per second incident on the surface of the...
- 17M.2.HL.TZ1.9a: Explain how each observation provides support for the particle theory but not the wave theory...
-
17M.2.HL.TZ2.7a.i:
Calculate the wavelength of the light.
-
18M.2.HL.TZ2.9b:
Bohr modified the Rutherford model by introducing the condition mvr = n. Outline the reason for this modification.
-
17M.2.HL.TZ1.9b.i:
Determine a value for Planck’s constant.
-
17M.2.HL.TZ2.7b:
Radiation of photon energy 5.2 x 10–19 J is now incident on the photocell. Calculate the maximum velocity of the emitted electrons.
-
18M.2.HL.TZ2.9c.ii:
Using the answer in (b) and (c)(i), deduce that the radius r of the electron’s orbit in the ground state of hydrogen is given by the following expression.
-
21M.2.HL.TZ1.10c:
The photoelectrons are emitted from a sodium surface. Sodium has a work function of 2.3 eV.
Calculate the wavelength of the radiation incident on the sodium. State an appropriate unit for your answer.
-
21M.2.HL.TZ2.9c:
Photons with energy 1.1 × 10−18 J are incident on a third metal surface. The maximum energy of electrons emitted from the surface of the metal is 5.1 × 10−19 J.
Calculate, in eV, the work function of the metal.
-
21N.1.HL.TZ0.37:
In a photoelectric experiment a stopping voltage required to prevent photoelectrons from flowing across the photoelectric cell is measured for light of two frequencies and . The results obtained are shown.
The ratio is an estimate of
A.B.
C.
D.
- 21N.2.HL.TZ0.4d.i: State and explain what happens to the kinetic energy of an emitted photoelectron.

