| Date | May 2018 | Marks available | 1 | Reference code | 18M.3.sl.TZ1.2 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Annotate | Question number | 2 | Adapted from | N/A |
Question
Students were asked to investigate how a change in concentration of hydrochloric acid, HCl, affects the initial rate of its reaction with marble chips, CaCO3.
They decided to measure how long the reaction took to complete when similar chips were added to 50.0 cm3 of 1.00 mol dm−3 acid and 50.0 cm3 of 2.00 mol dm−3 acid.
Two methods were proposed:
(1) using small chips, keeping the acid in excess, and recording the time taken for the solid to disappear
(2) using large chips, keeping the marble in excess, and recording the time taken for bubbles to stop forming.
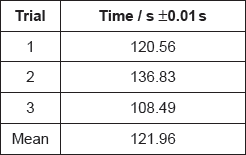
A group recorded the following results with 1.00 mol dm−3 hydrochloric acid:
Annotate the balanced equation below with state symbols.
CaCO3(__) + 2HCl(__) → CaCl2(__) + CO2(__) + H2O(__)
Neither method actually gives the initial rate. Outline a method that would allow the initial rate to be determined.
Deduce, giving a reason, which of the two methods would be least affected by the chips not having exactly the same mass when used with the different concentrations of acid.
State a factor, that has a significant effect on reaction rate, which could vary between marble chips of exactly the same mass.
Justify why it is inappropriate to record the uncertainty of the mean as ±0.01 s.
If doubling the concentration doubles the reaction rate, suggest the mean time you would expect for the reaction with 2.00 mol dm−3 hydrochloric acid.
Another student, working alone, always dropped the marble chips into the acid and then picked up the stopwatch to start it. State, giving a reason, whether this introduced a random or systematic error.
Markscheme
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
Accept “CO2(aq)”.
[1 mark]
measure the volume of gas at different times «plot a graph and extrapolate»
OR
measure the mass of the reaction mixture at different times «plot a graph and extrapolate»
Accept other techniques that yield data which can be plotted and extrapolated.
[1 mark]
method 2 AND marble is in excess «so a little extra has little effect»
OR
large chips AND marble is in excess «so a little extra has little effect»
OR
method 2 AND HCl is limiting reagent «so a little extra marble has little effect»
OR
large chips AND HCl is limiting reagent «so a little extra marble has little effect»
Accept, as a reason, that “as the mass is greater the percentage variation will be lower”.
[1 mark]
surface area
OR
purity «of the marble»
Accept “shape of the chip”.
[1 mark]
variation of individual values is much greater «than this uncertainty»
OR
«uncertainty» does not take into account «student» reaction time
[1 mark]
« = 60.98 s» = 61 «s»
[1 mark]
systematic AND always makes the time shorter «than the actual value»
OR
systematic AND it is an error in the method used «not an individual measurement»
OR
systematic AND more repetitions would not reduce the error
Accept, as reason, “it always affects the value in the same direction” OR “the error is consistent”.
[1 mark]

