| Date | May 2017 | Marks available | 1 | Reference code | 17M.2.hl.TZ1.3 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Formulate | Question number | 3 | Adapted from | N/A |
Question
Vanadium has a number of different oxidation states.
Electrode potentials for the reactions of vanadium and other species are shown below.
Determine the oxidation state of vanadium in each of the following species.
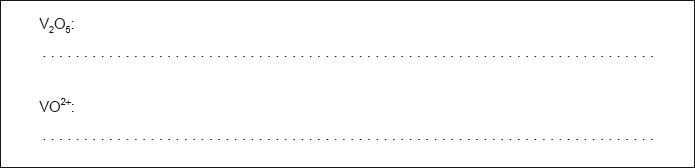
Identify, from the table, a non-vanadium species that can reduce VO2+(aq) to V3+(aq) but no further.
Identify, from the table, a non-vanadium species that could convert to V2+(aq).
Formulate an equation for the reaction between VO2+(aq) and V2+(aq) in acidic solution to form V3+(aq).
Comment on the spontaneity of this reaction by calculating a value for using the data given in (b) and in section 1 of the data booklet.
Markscheme
Do not penalize incorrect notation twice.
[2 marks]
H2SO3(aq)
OR
Pb(s)
[1 mark]
Zn(s)
[1 mark]
Accept equilibrium sign.
[1 mark]
spontaneous as is negative
Do not award M3 as a stand-alone answer.
Accept “spontaneous” for M3 if answer given for M2 is negative.
Accept “spontaneous as is positive” for M3.
[3 marks]
Examiners report
Syllabus sections
-
18M.1.sl.TZ1.23:
Which coefficients correctly balance this redox equation?
aFe2+(aq) + MnO4−(aq) + bH+(aq) → cFe3+(aq) + Mn2+(aq) + dH2O(l)

- 22M.1.sl.TZ1.21: In which of the following species would sulfur be reduced if converted to SCl2? A. ...
- 18M.1.sl.TZ2.22: Which can describe oxidation? A. Loss of hydrogen B. Decrease in oxidation...
- 18M.1.sl.TZ2.21: Which element has the same oxidation number in both species? A. C in C2H4 and CO2 B. ...
-
17M.2.sl.TZ1.3a:
Determine the oxidation state of vanadium in each of the following species.
-
18N.2.sl.TZ0.3d.i:
Bromate(V) ions act as oxidizing agents in acidic conditions to form bromide ions.
Deduce the half-equation for this reduction reaction.
-
17M.2.sl.TZ2.2a.ii:
Deduce the overall redox equation for the reaction between acidic Sn2+(aq) and Cr2O72–(aq), using section 24 of the data booklet.
- 17N.2.sl.TZ0.2e.i: Identify the strongest reducing agent in the given list.
-
17N.2.hl.TZ0.7d:
Identify the best reducing agent in the table above.
- 22M.2.sl.TZ2.1c: The reaction of lithium with water is a redox reaction. Identify the oxidizing agent in the...
- 22M.2.sl.TZ1.1d(ii): Determine the oxidation state of nitrogen in Mg3N2 and in NH3.
-
22M.2.sl.TZ1.1d(iii):
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
- 22M.2.hl.TZ1.1d(iii): Determine the oxidation state of nitrogen in Mg3N2 and in NH3.
-
22M.2.hl.TZ1.1d(iv):
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
-
17M.2.sl.TZ1.1a.i:
Suggest how the change of iodine concentration could be followed.
- 16N.2.sl.TZ0.1b: Determine the average oxidation state of carbon in ethene and in...
-
20N.2.hl.TZ0.4d(iv):
Suggest a metal that could replace nickel in a new half-cell and reverse the electron flow. Use section 25 of the data booklet.
-
17N.1.sl.TZ0.22:
Which of the following is a redox reaction?
A. 3Mg (s) + 2AlCl3 (aq) → 2Al (s) + 3MgCl2 (aq)
B. SiO2 (s) + 2NaOH (aq) → Na2SiO3 (aq) + H2O (l)
C. KCl (aq) + AgNO3 (aq) → AgCl (s) + KNO3 (aq)
D. 2NaHCO3 (aq) → Na2CO3 (aq) + CO2 (g) + H2O (l)
- 17M.1.hl.TZ2.36: Which compounds can be reduced? I. C2H4II. CH3COOHIII. CH3CHO A. I and II...
- 17M.1.hl.TZ1.28: Which change represents oxidation? A. HClO4 to HClO3 B. N2 to NH3 C. N2O to...
-
18M.1.sl.TZ1.21:
Which equation shows oxygen undergoing reduction?
A. 2F2 + O2 → 2F2O
B. Na2O + H2O → 2NaOH
C. H2O2 + 2HI → 2H2O + I2
D. 2CrO42− + 2H+ Cr2O72− + H2O
- 19M.1.hl.TZ2.28: Which compound contains sulfur with an oxidation state of +6? A. SO2 B. H2S C. H2SO3 D....
-
17M.1.sl.TZ2.22:
Which of the following is not a redox reaction?
A. CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)
B. C(s) + O2(g) → CO2(g)
C. 2CO(g) → CO2(g) + C(s)
D. CH3COOH(aq) + NaOH(aq) → CH3COONa(aq) + H2O(l)
-
17M.2.sl.TZ2.2a.i:
State the oxidation half-equation.
-
22M.2.hl.TZ2.3b(i):
Molten zinc chloride undergoes electrolysis in an electrolytic cell at 450 °C.
Deduce the half-equations for the reaction at each electrode.
-
22M.2.hl.TZ2.5b:
Calculate the oxidation state of sulfur in iron(II) disulfide, FeS2.
-
17M.1.sl.TZ2.19:
Which of the following does not react with dilute HCl(aq)?
A. Na2CO3
B. Cu
C. Zn
D. CuO
-
17M.2.hl.TZ1.4d:
Suggest why experiments involving tetracarbonylnickel are very hazardous.
-
17M.2.sl.TZ2.4a.iii:
Determine the oxidation state of nitrogen in the two reactants.
-
18N.1.sl.TZ0.21:
Which is correct for the reaction?
P4 (s) + 3H2O (l) + 3OH− (aq) → PH3 (g) + 3H2PO2− (aq)
- 18N.1.sl.TZ0.23: Which represents a reduction? A. SO3 to SO42− B. Mn2O3 to MnO2 C. H2O2 to...
- 18N.2.sl.TZ0.2d.ii: Deduce the average oxidation state of carbon in propan-2-ol.
-
18N.2.hl.TZ0.3d.i:
Bromate(V) ions act as oxidizing agents in acidic conditions to form bromide ions.
Deduce the half-equation for this reduction reaction.
- 18N.2.hl.TZ0.6a.iii: Deduce the average oxidation state of carbon in butanoic acid.
-
19M.2.sl.TZ2.4c(i):
State the name of this compound, applying IUPAC rules.
-
18N.2.hl.TZ0.3d.ii:
Bromate(V) ions oxidize iron(II) ions, Fe2+, to iron(III) ions, Fe3+.
Deduce the equation for this redox reaction.
- 21N.2.sl.TZ0.6a: Outline what is measured by BOD.
-
19M.2.sl.TZ1.3e:
State the oxidation number of carbon in sodium carbonate, Na2CO3.
-
19M.2.hl.TZ1.3k:
State the oxidation number of carbon in sodium carbonate, Na2CO3.
-
19M.2.hl.TZ2.1d(ii):
Deduce the average oxidation state of carbon in product B.
-
19M.1.sl.TZ1.22:
Which is the species oxidized and the oxidizing agent in the reaction?
MnO2 (s) + 4HCl (aq) → MnCl2 (aq) + Cl2 (g) + 2H2O (l)
- 19M.1.sl.TZ2.21: Which species contains nitrogen with the highest oxidation state? A. NO3− B. NO2− C....
-
17N.1.sl.TZ0.21:
What are the oxidation states of chromium in (NH4)2Cr2O7 (s) and Cr2O3 (s)?
-
19M.2.sl.TZ1.4b(iv):
MnO2 is another possible catalyst for the reaction. State the IUPAC name for MnO2.
-
21N.2.sl.TZ0.6c(iv):
The three steps of the Winkler Method are redox reactions.
Deduce the reduction half-equation for step II.
-
22M.1.sl.TZ2.11:
What is the name of the compound with formula Ti3(PO4)2?
A. Titanium phosphate
B. Titanium(II) phosphate
C. Titanium(III) phosphate
D. Titanium(IV) phosphate
-
16N.1.sl.TZ0.17:
Which experimental methods could be used to observe the progress of the following reaction?
Cr2O72-(aq) + 6I-(aq) + 14H+(aq) → 2Cr3+(aq) + 3I2(aq) + 7H2O(l)
I. Change in colour
II. Change in mass
III. Change in electrical conductivityA. I and II only
B. I and III only
C. II and III only
D. I, II and III
- 16N.3.sl.TZ0.1c: Despite widespread improvements in the provision of safe drinking water, the sale of bottled...
-
17M.2.hl.TZ1.3b.i:
Identify, from the table, a non-vanadium species that can reduce VO2+(aq) to V3+(aq) but no further.
-
19M.2.hl.TZ2.4d(i):
State the name of this compound, applying IUPAC rules.
-
19M.2.hl.TZ2.4c:
Describe how the relative reactivity of rhenium, compared to silver, zinc, and copper, can be established using pieces of rhenium and solutions of these metal sulfates.
-
17M.1.hl.TZ2.28:
Which element is reduced in the following decomposition?
(NH4)2Cr2O7(s) → N2(g) + Cr2O3(s) + 4H2O(g)
A. N
B. H
C. Cr
D. O
- 19N.3.sl.TZ0.2b: Deduce, giving your reason, which catalyst is most effective at fully oxidizing ethanol.
- 19N.3.sl.TZ0.2a(iii): List the three products at the anode from the least to the most oxidized.
-
19N.3.sl.TZ0.5a:
Discuss why different methods of reduction are needed to extract metals.
-
17M.3.sl.TZ2.5b.ii:
Deduce the redox equation for the reaction of nickel(II) chloride solution with the metal identified in (b)(i).
-
19M.2.hl.TZ1.4b(v):
MnO2 is another possible catalyst for the reaction. State the IUPAC name for MnO2.
-
19M.2.sl.TZ2.4b:
Describe how the relative reactivity of rhenium, compared to silver, zinc, and copper, can be established using pieces of rhenium and solutions of these metal sulfates.
-
17M.1.sl.TZ1.21:
What is the order of decreasing reactivity of the metals (most reactive first)?
Zn(s) + Sn2+(aq) → Zn2+(aq) + Sn(s)
Cu(s) + Zn2+(aq) → No Reaction
Sn(s) + Cu2+(aq) → Sn2+(aq) + Cu(s)
Ag(s) + Cu2+(aq) → No ReactionA. Zn > Cu > Sn > Ag
B. Sn > Zn > Ag > Cu
C. Ag > Cu > Zn > Sn
D. Zn > Sn > Cu > Ag
-
17M.3.sl.TZ2.5b.i:
Nickel is also used as a catalyst. It is processed from an ore until nickel(II) chloride solution is obtained. Identify one metal, using sections 24 and 25 of the data booklet, which will not react with water and can be used to extract nickel from the solution.
-
17M.2.sl.TZ1.3b:
Formulate an equation for the reaction between VO2+(aq) and V2+(aq) in acidic solution to form V3+(aq).
-
17M.1.hl.TZ1.29:
A reaction takes place when a rechargeable battery is used:
Pb(s) + PbO2(s) + 4H+(aq) + 2SO42−(aq) → 2PbSO4(s) + 2H2O(l)
Which statements are correct?
I. H+ is reduced
II. The oxidation state of Pb metal changes from 0 to +2
III. PbO2 is the oxidising agentA. I and II only
B. I and III only
C. II and III only
D. I, II and III
-
17M.2.hl.TZ1.3b.ii:
Identify, from the table, a non-vanadium species that could convert to V2+(aq).
-
17M.1.sl.TZ1.22:
What is the oxidation half-equation in the redox reaction?
2S2O32–(aq) + I2(aq) → S4O62–(aq) + 2I–(aq)
A. I2(aq) + 2e– → 2I–(aq)
B. 2I–(aq) → I2(aq) + 2e–
C. 2S2O32–(aq) → S4O62–(aq) + 2e–
D. S4O62–(aq) + 2e– → 2S2O32–(aq)
-
17M.1.sl.TZ2.21:
Which element is reduced in the following decomposition?
(NH4)2Cr2O7(s) → N2(g) + Cr2O3(s) + 4H2O(g)
A. N
B. H
C. Cr
D. O
-
17M.2.hl.TZ1.4a:
Formulate an equation for the oxidation of nickel(II) sulfide to nickel(II) oxide.
-
21N.1.sl.TZ0.22:
What is the change in the oxidation state of oxygen?
2Fe2+ (aq) + H2O2 (aq) + 2H+ (aq) → 2H2O (l) + 2Fe3+ (aq)
A. +1B. 0
C. −1
D. −2
- 19N.1.sl.TZ0.22: In which species does sulfur have the same oxidation state as in SO32–? A. S2O32– B. ...
-
16N.2.hl.TZ0.4i:
Magnesium chloride can be electrolysed.
(i) Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922K and 987K respectively.
(ii) Identify the type of reaction occurring at the cathode (negative electrode).
(iii) State the products when a very dilute aqueous solution of magnesium chloride is electrolysed.
-
17M.2.sl.TZ2.4a.iv:
Deduce, giving a reason, which species is the reducing agent.
- 19N.3.sl.TZ0.2a(ii): Determine the change in the average oxidation state of carbon. From ethanol to...
-
18N.2.sl.TZ0.3d.ii:
Bromate(V) ions oxidize iron(II) ions, Fe2+, to iron(III) ions, Fe3+.
Deduce the equation for this redox reaction.
-
19M.2.sl.TZ2.1d(ii):
Deduce the average oxidation state of carbon in product B.
-
22M.1.sl.TZ1.23:
How many electrons are needed when the following half-equation is balanced using the lowest possible whole numbers?
__ NO3– (aq) + __ H+ (aq) + __ e– → __ NO (g) + __ H2O (l)
A. 1B. 2
C. 3
D. 5
- 22M.1.sl.TZ2.21: Which species could be reduced to form NO2? A. N2 B. NO3− C. HNO2 D. NO
-
19M.2.hl.TZ1.2g:
The combustion reaction in (f)(ii) can also be classed as redox. Identify the atom that is oxidized and the atom that is reduced.
-
19M.2.hl.TZ2.4e(ii):
Deduce the coefficients required to complete the half-equation.
ReO4− (aq) + ____H+ (aq) + ____e− ⇌ [Re(OH)2]2+ (aq) + ____H2O (l) Eθ = +0.36 V
-
16N.2.sl.TZ0.4g:
Magnesium chloride can be electrolysed.
Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922 K and 987 K respectively.
Anode (positive electrode):
Cathode (negative electrode):
-
20N.2.hl.TZ0.1b(vi):
Deduce, referring to oxidation states, whether is an oxidizing or reducing agent.
-
18M.2.hl.TZ2.4a:
In acidic solution, bromate ions, BrO3−(aq), oxidize iodide ions, I−(aq).
BrO3−(aq) + 6H+(aq) + 6e− Br−(aq) + 3H2O(l)
2I−(aq) I2(s) + 2e−
Formulate the equation for the redox reaction.
- 21M.1.sl.TZ1.21: A student performed displacement reactions using metals W and X and solutions of salts of...
-
21M.1.sl.TZ1.22:
What is correct for this redox reaction?
MnO2 (s) + 2− (aq) + 4H+ (aq) → Mn2+ (aq) + 2 (aq) + 2H2O (l)
-
21N.2.hl.TZ0.6c(iv):
The three steps of the Winkler Method are redox reactions.
Deduce the reduction half-equation for step II.
-
16N.1.sl.TZ0.21:
Which is a correct statement for the reaction below?
2MnO4-(aq) + 6H+(aq) + 5NO2-(aq) → 2Mn2+(aq) + 5NO3-(aq) + 3H2O(l)
A. MnO4- is the reducing agent and the oxidation number of Mn increases.
B. MnO4- is the oxidizing agent and the oxidation number of Mn decreases.
C. NO2- is the reducing agent and the oxidation number of N decreases.
D. NO2- is the oxidizing agent and the oxidation number of N increases. - 21M.2.hl.TZ1.1e(ii): Deduce the change in the oxidation state of sulfur.
- 21M.2.hl.TZ1.4b: The hydrogen peroxide could cause further oxidation of the methanol. Suggest a possible...
- 21M.2.sl.TZ1.1d(ii): Deduce the change in the oxidation state of sulfur.
-
20N.2.sl.TZ0.4d(ii):
Suggest a metal that could replace nickel in a new half-cell and reverse the electron flow. Use section 25 of the data booklet.
-
19N.1.sl.TZ0.23:
The following occurs when metal X is added to Y sulfate solution and Z sulfate solution. (X, Y and Z represent metal elements but not their symbols.)
X (s) + YSO4 (aq) → XSO4 (aq) + Y (s)
X (s) + ZSO4 (aq): no reactionWhat is the order of increasing reactivity?
A. X < Y < Z
B. Y < X < Z
C. Z < Y < X
D. Z < X < Y
-
17M.2.hl.TZ1.4b:
The nickel obtained from another ore, nickeliferous limonite, is contaminated with iron. Both nickel and iron react with carbon monoxide gas to form gaseous complexes, tetracarbonylnickel, , and pentacarbonyliron, . Suggest why the nickel can be separated from the iron successfully using carbon monoxide.
-
21M.2.sl.TZ1.3d(i):
Write the half-equation for the reduction of hydrogen peroxide to water in acidic solution.
- 21M.2.sl.TZ1.4b: The hydrogen peroxide could cause further oxidation of the methanol. Suggest a possible...
-
21M.2.sl.TZ1.3d(ii):
Deduce a balanced equation for the oxidation of Fe2+ by acidified hydrogen peroxide.
- 20N.1.sl.TZ0.23: What are the oxidation states of oxygen?
-
20N.2.sl.TZ0.1b(vi):
Deduce, referring to oxidation states, whether is an oxidizing or reducing agent.
-
20N.2.sl.TZ0.1b(v):
State the oxidation state of manganese in and .
-
20N.2.hl.TZ0.1b(v):
State the oxidation state of manganese in and .
-
19M.3.hl.TZ1.1b(iii):
Compare the ease of oxidation of s-block and d-block metals to their melting points and densities. Use section 25 of the data booklet.
-
17N.1.hl.TZ0.30:
Consider the following half-equations:
I2 (s) + 2e– 2I– (aq) Eθ = +0.54 V
(brown) (colourless)MnO4– (aq) + 8H+ (aq) + 5e– Mn2+ (aq) + 4H2O (l) Eθ = +1.51 V
(purple) (colourless)Which statement is correct for the reaction between KMnO4 (aq) and KI (aq) in acidic conditions?
A. MnO4– reduces I– to I2.
B. I– reduces MnO4– to Mn2+.
C. The colour changes from brown to purple.
D. MnO4– is oxidized to Mn2+.
-
22M.2.sl.TZ2.5a:
Deduce the half-equations for the reaction at each electrode.
-
22M.2.sl.TZ2.5b:
Deduce the overall cell reaction including state symbols. Use section 7 of the data booklet.
- 21M.1.sl.TZ2.21: What is the oxidation state of oxygen in H2O2? A. −2 B. −1 C. +1 D. +2
-
22M.2.hl.TZ1.2a:
Suggest an experiment that shows that magnesium is more reactive than zinc, giving the observation that would confirm this.
-
19M.3.sl.TZ1.1b(iii):
Compare the ease of oxidation of s-block and d-block metals to their melting points and densities. Use section 25 of the data booklet.
-
22M.2.sl.TZ1.3b:
Suggest an experiment that shows that magnesium is more reactive than zinc, giving the observation that would confirm this.
-
22M.1.sl.TZ2.22:
Which combination best describes what is happening to chloromethane, CH3Cl, in the equation below?
CH3Cl (g) + H2 (g) CH4 (g) + HCl (g)
A. Oxidation and addition
B. Oxidation and substitution
C. Reduction and addition
D. Reduction and substitution
-
22M.2.hl.TZ2.3b(ii):
Deduce the overall cell reaction including state symbols. Use section 7 of the data booklet.
- 21N.2.hl.TZ0.6a: Outline what is measured by BOD.

