| Date | May 2017 | Marks available | 3 | Reference code | 17M.2.sl.TZ1.5 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Formulate | Question number | 5 | Adapted from | N/A |
Question
This question is about carbon and chlorine compounds.
Ethane, C2H6, reacts with chlorine in sunlight. State the type of this reaction and the name of the mechanism by which it occurs.
Formulate equations for the two propagation steps and one termination step in the formation of chloroethane from ethane.
One possible product, X, of the reaction of ethane with chlorine has the following composition by mass:
carbon: 24.27%, hydrogen: 4.08%, chlorine: 71.65%
Determine the empirical formula of the product.
The mass and 1HNMR spectra of product X are shown below. Deduce, giving your reasons, its structural formula and hence the name of the compound.
Chloroethene, C2H3Cl, can undergo polymerization. Draw a section of the polymer with three repeating units.
Markscheme
substitution AND «free-»radical
OR
substitution AND chain
Award [1] for “«free-»radical substitution” or “SR” written anywhere in the answer.
[1 mark]
Two propagation steps:
C2H6 + •Cl → C2H5• + HCl
C2H5• + Cl2 → C2H5Cl + •Cl
One termination step:
C2H5• + C2H5• → C4H10
OR
C2H5• + •Cl → C2H5Cl
OR
•Cl + •Cl → Cl2
Accept radical without • if consistent throughout.
Allow ECF from incorrect radicals produced in propagation step for M3.
[3 marks]
= 2.021 AND = 4.04 AND
«hence» CH2Cl
Accept : :
Do not accept C2H4Cl2.
Award [2] for correct final answer.
[2 marks]
molecular ion peak(s) «about» m/z 100 AND «so» C2H4Cl2 «isotopes of Cl»
two signals «in 1HNMR spectrum» AND «so» CH3CHCl2
OR
«signals in» 3:1 ratio «in 1HNMR spectrum» AND «so» CH3CHCl2
OR
one doublet and one quartet «in 1HNMR spectrum» AND «so» CH3CHCl2
1,1-dichloroethane
Accept “peaks” for “signals”.
Allow ECF for a correct name for M3 if an incorrect chlorohydrocarbon is identified
[3 marks]
Continuation bonds must be shown.
Ignore square brackets and “n”.
Accept 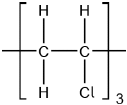 .
.
Accept other versions of the polymer, such as head to head and head to tail.
Accept condensed structure provided all C to C bonds are shown (as single).
[1 mark]
Examiners report
Syllabus sections
- 18M.1.sl.TZ1.25: What is the product of the reaction between hex-3-ene and steam? A. Hexan-1-ol B. ...
- 18M.1.sl.TZ1.26: Which of these reactions proceeds by a free radical mechanism in the presence of UV...
-
18M.2.sl.TZ1.3a.ii:
Outline the formation of polyethene from ethene by drawing three repeating units of the polymer.
-
18M.2.sl.TZ1.3d:
State the characteristic reaction mechanism of benzene.
- 18M.1.sl.TZ2.26: What is the mechanism for the reaction of propene with iodine in the dark? A. ...
- 22M.2.hl.TZ1.5b(iii): Deduce the structural formula of the repeating unit of the polymer formed from this alkene.
-
22M.2.hl.TZ1.5c:
Deduce what would be observed when Compound B is warmed with acidified aqueous potassium dichromate (VI).
-
22M.1.sl.TZ1.26:
Which reagents and conditions are best for converting propan-1-ol into propanoic acid?
A. Reflux with acidified potassium dichromate (VI)
B. Reflux with aqueous sodium hydroxide
C. Distil with acidified potassium dichromate (VI)
D. Distil with aqueous sodium hydroxide
- 22M.1.sl.TZ1.27: What is produced when chlorobutane is treated with aqueous sodium hydroxide solution? A. ...
- 22M.2.sl.TZ1.3f(i): Identify the type of reaction.
-
22M.2.sl.TZ1.3e:
Deduce what would be observed when Compound B is warmed with acidified aqueous potassium dichromate (VI).
- 19N.2.sl.TZ0.3e: Ethene is often polymerized. Draw a section of the resulting polymer, showing two repeating...
-
22M.1.hl.TZ1.34:
Which reagents and conditions are best for converting propan-1-ol into propanoic acid?
A. Reflux with acidified potassium dichromate (VI)
B. Reflux with LiAlH4
C. Distil with acidified potassium dichromate (VI)
D. Distil with LiAlH4
-
19N.2.sl.TZ0.3c(i):
Write an equation for the complete combustion of the organic product in (b).
-
17M.3.hl.TZ2.3c.ii:
Suggest, giving one reason, whether this is an addition or condensation reaction.
- 22M.1.sl.TZ2.27: Which reaction mechanisms are typical for alcohols and halogenoalkanes?
-
18M.2.sl.TZ2.7b.ii:
The aldehyde can be further oxidized to a carboxylic acid.
Outline how the experimental procedures differ for the synthesis of the aldehyde and the carboxylic acid.
- 18M.1.hl.TZ2.35: Which is the correct combination of substitution reaction mechanisms?
-
22M.1.hl.TZ2.35:
Which reaction involves homolytic fission?
A. CH4 + Cl2
B. CH3Br + NaOH
C. (CH3)3CBr + NaOH
D. C6H6 + HNO3 + H2SO4
- 18M.1.hl.TZ1.33: Which monomer could create this polymer? A. But-2-ene B. But-1-ene C. ...
- 17M.1.sl.TZ2.26: Which conditions are used to convert ethanol to ethanal? A. Excess oxidizing agent and...
-
22M.2.sl.TZ2.4d(ii):
Write the equation for the reaction between but-2-ene and hydrogen bromide.
-
22M.2.sl.TZ2.4d(iii):
State the type of reaction.
-
17M.2.sl.TZ1.5d:
Chloroethene, C2H3Cl, can undergo polymerization. Draw a section of the polymer with three repeating units.
- 17M.1.sl.TZ2.25: Which describes the reaction between a halogen and ethane?
-
17M.2.hl.TZ2.6b:
The overall equation for monochlorination of methane is:
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)
Calculate the standard enthalpy change for the reaction, ΔH θ, using section 12 of the data booklet.
-
19M.1.hl.TZ2.35:
What must be present on a nucleophile?
A. Negative charge
B. Lone pair of electrons
C. Positive charge
D. Symmetrical distribution of electrons
-
19M.1.sl.TZ1.27:
Which alcohol would produce a carboxylic acid when heated with acidified potassium dichromate(VI)?
A. propan-2-ol
B. butan-1-ol
C. 2-methylpropan-2-ol
D. pentan-3-ol
- 19N.1.sl.TZ0.26: What type of reaction occurs when C6H13Br becomes C6H13OH? A. Nucleophilic...
- 18M.1.hl.TZ1.40: Which would be the most effective method to distinguish between liquid propan-1-ol and...
-
18M.2.hl.TZ1.3a.ii:
Outline the formation of polyethene from ethene by drawing three repeating units of the polymer.
-
20N.2.hl.TZ0.1d(i):
State the type of reaction occurring when ethane reacts with chlorine to produce chloroethane.
-
18M.2.hl.TZ1.3a.i:
Ethyne, like ethene, undergoes hydrogenation to form ethane. State the conditions required.
- 22M.2.hl.TZ2.8d(iii): State the type of reaction.
-
22M.2.hl.TZ2.8d(ii):
Write the equation for the reaction between but-2-ene and hydrogen bromide.
- 16N.3.sl.TZ0.17b: Oseltamivir does not possess the carboxyl group needed for activity until it is chemically...
-
18N.1.sl.TZ0.27:
Which compounds react to form CH3CH2CH2COOCH(CH3)2?
A. propanoic acid and propan-2-ol
B. propanoic acid and butan-2-ol
C. butanoic acid and propan-1-ol
D. butanoic acid and propan-2-ol
-
18N.2.sl.TZ0.2d.i:
State a suitable oxidizing agent for the oxidation of propan-2-ol in an acidified aqueous solution.
-
18N.2.sl.TZ0.2d.iii:
Deduce the product of the oxidation of propan-2-ol with the oxidizing agent in (d)(i).
- 18N.2.hl.TZ0.9b: Classify 1-bromopropane as a primary, secondary or tertiary halogenoalkane, giving a reason.
- 18N.2.hl.TZ0.9a: State a reason why most halogenoalkanes are more reactive than alkanes.
- 18N.2.sl.TZ0.7a: Identify the type of reaction in step 1.
-
19M.2.sl.TZ1.1c(i):
Identify the initiation step of the reaction and its conditions.
-
17M.3.hl.TZ1.9a:
Deduce the repeating unit of the polymer and the other product of the reaction.
-
18M.2.sl.TZ2.7b.i:
Formulate the ionic equation for the oxidation of propan-1-ol to the corresponding aldehyde by acidified dichromate(VI) ions. Use section 24 of the data booklet.
-
19N.2.sl.TZ0.3b:
Write an equation for the reaction of C2H5Cl with aqueous sodium hydroxide to produce a C2H6O compound, showing structural formulas.
-
19M.1.hl.TZ1.34:
Which alcohol would produce a carboxylic acid when heated with acidified potassium dichromate(VI)?
A. propan-2-ol
B. butan-1-ol
C. 2-methylpropan-2-ol
D. pentan-3-ol
-
16N.2.sl.TZ0.5b:
Both propane and propene react with bromine.
(i) State an equation and the condition required for the reaction of 1 mol of propane with 1 mol of bromine.
(ii) State an equation for the reaction of 1 mol of propene with 1 mol of bromine.
(iii) State the type of each reaction with bromine.
Propane:
Propene:
-
16N.2.sl.TZ0.1d:
Ethane-1,2-diol can be oxidized first to ethanedioic acid, (COOH)2, and then to carbon dioxide and water. Suggest the reagents to oxidize ethane-1,2-diol.
-
18N.1.sl.TZ0.24:
Which compounds cause the colour of acidified potassium manganate(VII) solution to change from purple to colourless?
I. CH3CH2CH2CH2OH
II. (CH3)3CCH2OH
III. CH3CH2CH(OH)CH3
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
-
19M.2.hl.TZ2.1c(i):
State the name of product B, applying IUPAC rules.
- 19M.1.sl.TZ1.26: What is the mechanism of the reaction between alkenes and halogens in the absence of...
-
19M.1.sl.TZ2.26:
Methane reacts with chlorine in sunlight.
CH4 (g) + Cl2 (g) → CH3Cl (g) + HCl (g)
Which type of reaction occurs?
A. free-radical substitution
B. electrophilic substitution
C. nucleophilic substitution
D. electrophilic addition
-
18M.2.hl.TZ1.7c.i:
State the organic product of the reaction between 1-chlorobutane, CH3CH2CH2CH2Cl, and aqueous sodium hydroxide.
- 19M.1.sl.TZ2.25: Which of the following can be both formed from bromoethane and converted directly into...
-
19M.2.hl.TZ2.1a:
Write an equation for the complete combustion of ethyne.
-
22M.1.sl.TZ2.25:
Which structure represents a repeating unit of a polymer formed from propene?
A. –CH2–CH(CH3)–
B. –CH2–CH2–CH2–
C. –CH(CH3)–CH(CH3)–
D. –CH2–CH2–
-
17N.2.sl.TZ0.6a.i:
Deduce the type of chemical reaction and the reagents used to distinguish between these compounds.
- 17M.1.hl.TZ1.35: What is the major product of the reaction between 2-methylbut-2-ene and hydrogen bromide? A....
- 17N.1.sl.TZ0.27: Which type of reaction occurs between an alcohol and a carboxylic acid? A. Addition B....
- 16N.1.sl.TZ0.26: Which type of reaction occurs when methanol and propanoic acid react together in the presence...
-
16N.1.sl.TZ0.25:
Which monomer is used to form the polymer with the following repeating unit?
A. CH3CH=CHCH3
B. CH3CH2CH=CH2
C. CH3CH2CH2CH3
D. (CH3)2C=CH2
- 16N.3.sl.TZ0.2b: In trial 3, the students noticed that after heating, the crucible had turned black on the...
-
19M.2.sl.TZ1.1c(ii):
1,4-dimethylbenzene reacts as a substituted alkane. Draw the structures of the two products of the overall reaction when one molecule of bromine reacts with one molecule of 1,4-dimethylbenzene.
-
17N.1.sl.TZ0.25:
Which compound can be oxidized when heated with an acidified solution of potassium dichromate(VI)?
A. CH3C(O)CH2CH3
B. CH3CH2CH(OH)CH3
C. (CH3)3COH
D. CH3(CH2)2COOH
- 17N.3.sl.TZ0.8b.i: State the type of reaction occurring during the titration.
-
17N.2.sl.TZ0.6b:
Explain, with the help of equations, the mechanism of the free-radical substitution reaction of ethane with bromine in presence of sunlight.
- 17N.1.sl.TZ0.24: What is the major product of the reaction between HCl and but-2-ene? A....
-
19M.2.hl.TZ1.1d(i):
Identify the initiation step of the reaction and its conditions.
- 19N.3.sl.TZ0.9d(i): State one similarity and one difference in composition between phospholipids and...
- 19N.2.sl.TZ0.3a: State the type of reaction which converts ethene into C2H5Cl.
- 19N.3.hl.TZ0.12c: State one similarity and one difference in composition between phospholipids and...
-
18M.1.sl.TZ1.27:
Which compound could be formed when CH3CH2CH2OH is heated with acidified potassium dichromate(VI)?
I. CH3CH2CHO
II. CH3CH2COOH
III. CH3COCH3
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
- 21N.2.sl.TZ0.4a(i): Identify the type of reaction.
- 19N.2.hl.TZ0.3a(i): State the type of reaction.
-
19N.2.hl.TZ0.3c(i):
Write an equation for the complete combustion of the compound C3H8O formed in (a)(iv).
- 19N.2.hl.TZ0.3e: Propene is often polymerized. Draw a section of the resulting polymer, showing two repeating...
-
17M.2.hl.TZ2.7b.ii:
Deduce the structural formula of the main organic product when hex-1-ene reacts with hydrogen bromide.
-
19N.3.hl.TZ0.5a:
Draw the structure of the monomers of Kevlar® if the by-product of the condensation polymerization is hydrogen chloride.
-
19N.3.sl.TZ0.14a:
Write the equation for the complete combustion of ethanol.
-
17M.2.sl.TZ1.6b:
State the typical reactions that benzene and cyclohexene undergo with bromine.
-
17M.2.sl.TZ1.6a:
Discuss the physical evidence for the structure of benzene.
-
18M.2.hl.TZ1.3b:
Ethyne reacts with chlorine in a similar way to ethene. Formulate equations for the following reactions.
-
18M.2.sl.TZ1.3a.i:
Ethyne, like ethene, undergoes hydrogenation to form ethane. State the conditions required.
-
19M.1.hl.TZ2.33:
Methane reacts with chlorine in sunlight.
CH4 (g) + Cl2 (g) → CH3Cl (g) + HCl (g)
Which type of reaction occurs?
A. free-radical substitution
B. electrophilic substitution
C. nucleophilic substitution
D. electrophilic addition
- 21M.2.hl.TZ1.5e(iv): Propan-2-ol can also be formed in one step from a compound containing a carbonyl...
-
17M.2.hl.TZ2.7b.i:
Bromine was added to hexane, hex-1-ene and benzene. Identify the compound(s) which will react with bromine in a well-lit laboratory.
-
19M.2.sl.TZ2.1a:
Write an equation for the complete combustion of ethyne.
-
17M.2.sl.TZ2.6c:
Polyvinyl chloride (PVC) is a polymer with the following structure.
State the structural formula for the monomer of PVC.
-
17M.2.sl.TZ1.5a:
Ethane, C2H6, reacts with chlorine in sunlight. State the type of this reaction and the name of the mechanism by which it occurs.
-
17M.2.sl.TZ2.6b:
Bromine was added to hexane, hex-1-ene and benzene. Identify the compound(s) which will react with bromine in a well-lit laboratory.
-
19N.2.hl.TZ0.3d(i):
State the reagents for the conversion of the compound C3H8O formed in (a)(iv) into C3H6O.
- 19N.1.sl.TZ0.28: Which compound cannot undergo addition polymerization?
- 19N.1.sl.TZ0.27: Which will react with a halogen by an electrophilic substitution mechanism?
- 19N.3.sl.TZ0.9b: State one impact on health of the increase in LDL cholesterol concentration in blood.
-
19M.2.hl.TZ2.6a:
Draw the repeating unit of polyphenylethene.
- 20N.1.sl.TZ0.25: Which molecule will decolorize bromine water in the dark? A. cyclohexane B. hexane C. ...
- 20N.1.sl.TZ0.27: Which mechanism does benzene most readily undergo? A. Nucleophilic substitution B. ...
-
17M.2.hl.TZ2.2a.v:
Identify one organic functional group that can react with acidified K2Cr2O7(aq).
- 22M.2.sl.TZ1.3d(ii): Deduce the structural formula of the repeating unit of the polymer formed from this alkene.
-
19M.2.sl.TZ2.1c(i):
Product A contains a carbon–carbon double bond. State the type of reactions that compounds containing this bond are likely to undergo.
-
19M.2.hl.TZ1.2f(ii):
Formulate the equation for the complete combustion of benzoic acid in oxygen using only integer coefficients.
-
19N.2.sl.TZ0.3d(i):
State the reagents and conditions for the conversion of the compound C2H6O, produced in (b), into C2H4O.
-
19M.2.hl.TZ1.1d(ii):
1,4-dimethylbenzene reacts as a substituted alkane. Draw the structures of the two products of the overall reaction when one molecule of bromine reacts with one molecule of 1,4-dimethylbenzene.
-
19N.3.hl.TZ0.24c:
Explain how redox chemistry is used to measure the ethanol concentration in a breathalyser.
- 21M.1.sl.TZ1.26: What is formed in a propagation step of the substitution reaction between bromine and...
-
21M.1.sl.TZ1.27:
Which monomer would produce the polymer shown?
A.
B.
C.
D.
- 21M.2.hl.TZ1.5d: A white solid was formed when ethene was subjected to high pressure. Deduce the type of...
- 21M.2.hl.TZ1.5c: Suggest two possible products of the incomplete combustion of ethene that would not be formed...
- 21M.2.hl.TZ1.5e(iii): 2-bromopropane can be converted directly to propan-2-ol. Identify the reagent required.
-
21M.1.sl.TZ2.26:
Which monomer forms the polymer shown?
A.
B.
C.
D.
-
19M.2.sl.TZ1.2b(ii):
Formulate the equation for the complete combustion of benzoic acid in oxygen using only integer coefficients.
-
19M.2.hl.TZ2.1d(i):
Suggest the reagents and conditions required to ensure a good yield of product B.
Reagents:
Conditions:
-
20N.2.sl.TZ0.1d(iii):
Write the equation for the reaction of chloroethane with a dilute aqueous solution of sodium hydroxide.
-
21M.1.hl.TZ2.37:
Which can be reduced to a secondary alcohol?
A. C2H5COOH
B. CH3CH2OCH3
C. (CH3)2CHCHO
D. CH3COC2H5
-
17N.2.sl.TZ0.6a.ii:
State the observation expected for each reaction giving your reasons.
- 21M.2.sl.TZ1.5d: A white solid was formed when ethene was subjected to high pressure. Deduce the type of...
- 21M.2.sl.TZ1.5c: Suggest two possible products of the incomplete combustion of ethene that would not be formed...
-
21M.2.sl.TZ2.4a:
Several compounds can be synthesized from but-2-ene. Draw the structure of the final product for each of the following chemical reactions.
-
21M.2.sl.TZ2.4d:
Oxidation of ethanol with potassium dichromate, K2Cr2O7, can form two different organic products. Determine the names of the organic products and the methods used to isolate them.
-
21M.2.hl.TZ2.4a:
Several compounds can be synthesized from but-2-ene. Draw the structure of the final product for each of the following chemical reactions.
-
21M.2.hl.TZ2.5c:
Write the equation and name the organic product when ethanol reacts with methanoic acid.
-
21M.2.hl.TZ2.5b:
Oxidation of ethanol with potassium dichromate, K2Cr2O7, can form two different organic products. Determine the names of the organic products and the methods used to isolate them.
-
17M.2.sl.TZ2.6a:
Using relevant equations, show the initiation and the propagation steps for this reaction.
-
20N.2.sl.TZ0.1d(iv):
Deduce the nucleophile for the reaction in d(iii).
-
20N.2.sl.TZ0.1d(i):
State the type of reaction occurring when ethane reacts with chlorine to produce chloroethane.
-
20N.2.hl.TZ0.1d(ii):
Predict, giving a reason, whether ethane or chloroethane is more reactive.
-
20N.2.sl.TZ0.1d(ii):
Predict, giving a reason, whether ethane or chloroethane is more reactive.
-
22M.2.hl.TZ2.8c:
Describe a test and the expected result to indicate the presence of carbon–carbon double bonds.
-
22M.2.sl.TZ2.4c:
Describe a test and the expected result to indicate the presence of carbon–carbon double bonds.
-
21M.1.sl.TZ2.27:
Which is a propagation step in the free-radical substitution mechanism of ethane with chlorine?
A. C2 → 2 •C
B. •C2H5 + C2 → C2H5C + •C
C. •C2H5 + •C → C2H5C
D. C2H6 + •C → C2H5C + •H
- 22M.2.hl.TZ1.5d(i): Identify the type of reaction.
-
20N.1.hl.TZ0.34:
Which molecule can be oxidized to a carboxylic acid by acidified potassium dichromate(VI)?
A. Propan-1-ol
B. Propan-2-ol
C. 2-methylpropan-2-ol
D. Propanone
-
22M.1.sl.TZ2.22:
Which combination best describes what is happening to chloromethane, CH3Cl, in the equation below?
CH3Cl (g) + H2 (g) CH4 (g) + HCl (g)
A. Oxidation and addition
B. Oxidation and substitution
C. Reduction and addition
D. Reduction and substitution
- 21N.2.sl.TZ0.4a(ii): Outline the role of the hydroxide ion in this reaction.
- 21N.2.hl.TZ0.10b(ii): State the type of reaction which occurs between but-1-ene and hydrogen iodide at room...
- 21N.2.sl.TZ0.7b: Formulate equations for the two propagation steps and one termination step in the formation...

