| Date | May 2014 | Marks available | 3 | Reference code | 14M.3.hl.TZ1.7 |
| Level | HL | Paper | 3 | Time zone | TZ1 |
| Command term | Compare and Draw | Question number | 7 | Adapted from | N/A |
Question
The most important components of nucleotides are the nitrogeneous bases, which consist of pyrimidines and purines.
Thymine (T), whose structure is given in Table 21 of the Data Booklet, is a pyrimidine.
Describe how thymine forms part of a nucleotide in deoxyribonucleic acid (DNA).
Adenine, A, whose structure is also given in Table 21 of the Data Booklet, is a purine found in DNA.
Draw the structure of the organic product formed from the condensation reaction of adenine with the sugar D-ribose (whose structure is given below) and identify the other product.
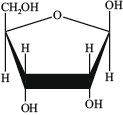
Structure of organic product:
Other product:
(i) Adenine (A), guanine (G), cytosine (C) and thymine (T) result in the double helix structure of DNA. Using the structures of adenine and thymine, draw a diagram to explain how thymine is able to play a role in forming a double helix.
(ii) Compare the bonding between cytosine and guanine with the bonding between adenine and thymine.
Markscheme
thymine (covalently) bonded to deoxyribose/pentose sugar / thymine bonds via a condensation reaction with sugar / N from thymine bonds to C on sugar / thymine joins with pentose sugar;
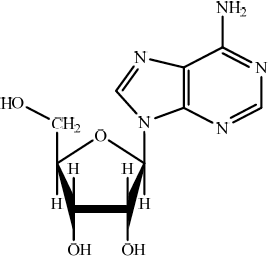 ;
;
\({{\text{H}}_{\text{2}}}{\text{O}}\)/water;
(i) 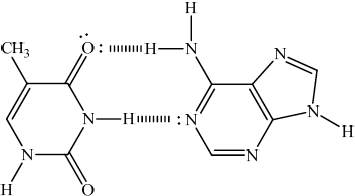
drawing correctly showing two hydrogen bonds;
Do not penalize structural error in bases.
Do not penalize if lone pairs are omitted.
(ii) both have hydrogen bonding;
C and G have three interactions (and A and T have two) / OWTTE;
Do not apply ECF here.
Examiners report
Parts of this question proved very difficult for candidates, in particular part (b) (most got water however) and (c) (i). In Question 7, only a tiny number of candidates were able to draw a correct structure of the nucleoside. Many students knew how many hydrogen bonds were between adenine and thymine but couldn't draw these bonds and often had no idea what hydrogen bonds were. In (d), most candidates suggested forensic and paternity cases as to possible uses of DNA profiling.
Parts of this question proved very difficult for candidates, in particular part (b) (most got water however) and (c) (i). In Question 7, only a tiny number of candidates were able to draw a correct structure of the nucleoside. Many students knew how many hydrogen bonds were between adenine and thymine but couldn't draw these bonds and often had no idea what hydrogen bonds were. In (d), most candidates suggested forensic and paternity cases as to possible uses of DNA profiling.
Parts of this question proved very difficult for candidates, in particular part (b) (most got water however) and (c) (i). In Question 7, only a tiny number of candidates were able to draw a correct structure of the nucleoside. Many students knew how many hydrogen bonds were between adenine and thymine but couldn't draw these bonds and often had no idea what hydrogen bonds were. In (d), most candidates suggested forensic and paternity cases as to possible uses of DNA profiling.

