| Date | May 2015 | Marks available | 4 | Reference code | 15M.2.hl.TZ1.6 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Determine | Question number | 6 | Adapted from | N/A |
Question
Bromomethane was used as a pesticide until it was found to be ozone-depleting.
State the equation for the reaction between methane and bromine to form bromomethane.
Explain, using equations, the complete free-radical mechanism for the reaction of methane with bromine, including necessary reaction conditions.
Bromomethane reacts with aqueous sodium hydroxide. State the organic product of this reaction.
Explain why the rate of the reaction between iodomethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{I}}\), and NaOH(aq) is faster than the rate of the reaction between \({\text{C}}{{\text{H}}_{\text{3}}}{\text{Br}}\) and NaOH(aq).
Bromine can be produced by the electrolysis of molten sodium bromide.
Deduce the half-equation for the reaction at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
Predict the products formed at the electrodes during the electrolysis of concentrated aqueous sodium bromide.
Positive electrode (anode):
Negative electrode (cathode):
Bromine reacts with aqueous sodium iodide.
\[{\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}} + {\text{2NaI(aq)}} \to {{\text{I}}_{\text{2}}}{\text{(aq)}} + {\text{2NaBr(aq)}}\]
Identify the oxidizing agent in this reaction.
Define the term standard electrode potential, \({E^\Theta }\).
Draw a labelled diagram for the voltaic cell in which the following reaction occurs.
\[{\text{Mg(s)}} + {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{Cu(s)}}\]
Include in your answer the direction of electron flow and the polarity of the electrodes.
A student measures a voltage of 2.65 V in the voltaic cell formed between magnesium and copper half-cells using a digital voltmeter.
State the random uncertainty of this value, in V, and the number of significant figures in the answer.
Random uncertainty:
Significant figures:
Outline how the student can reduce the random error in her results.
Determine the standard enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \), of NaCl(s), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using a Born-Haber cycle and tables 7, 10 and 13 of the data booklet. The standard enthalpy change of atomization (standard enthalpy change of sublimation), \(\Delta H_{{\text{at}}}^\Theta \), of Na(s) is \( + {\text{108 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Markscheme
\({\text{C}}{{\text{H}}_{\text{4}}} + {\text{B}}{{\text{r}}_{\text{2}}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{Br}} + {\text{HBr}}\);
Initiation:
\({\text{B}}{{\text{r}}_2}\xrightarrow{{{\text{UV/}}hf{\text{/}}hv}}{\text{2Br}} \bullet \);
Reference to UV/light or high temperatures must be included.
Propagation:
\({\text{Br}} \bullet + {\text{C}}{{\text{H}}_4} \to {\text{C}}{{\text{H}}_3} \bullet + {\text{HBr}}\);
\({\text{C}}{{\text{H}}_3} \bullet + {\text{B}}{{\text{r}}_2} \to {\text{C}}{{\text{H}}_3}{\text{Br}} + {\text{Br}} \bullet \);
Termination:
Award [1 max] for any one of:
\({\text{Br}} \bullet + {\text{Br}} \bullet \to {\text{B}}{{\text{r}}_2}\);
\({\text{C}}{{\text{H}}_3} \bullet + {\text{Br}} \bullet \to {\text{C}}{{\text{H}}_3}{\text{Br}}\);
\({\text{C}}{{\text{H}}_3} \bullet + {\text{C}}{{\text{H}}_3} \bullet \to {{\text{C}}_2}{{\text{H}}_6}\);
Allow representation of radical without \( \bullet \) (eg Br, \(C{H_{\text{3}}}\)) if consistent throughout mechanism.
Award [3 max] if initiation, propagation and termination are not stated or are incorrectly labelled for equations.
\({\text{methanol/C}}{{\text{H}}_{\text{3}}}{\text{OH}}\);
C–I bond is weaker than the C–Br bond so more easily broken;
C–I bond is longer than the C–Br bond / I larger than Br so bonding electrons not as tightly held / \({{\text{I}}^ - }\) is better leaving group than \({\text{B}}{{\text{r}}^ - }\);
Positive electrode (anode):
\({\text{2B}}{{\text{r}}^ - } \to {\text{B}}{{\text{r}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ - }{\text{/B}}{{\text{r}}^ - } \to \frac{1}{2}{\text{B}}{{\text{r}}_2}{\text{(g)}} + {{\text{e}}^ - }\);
Negative electrode (cathode):
\({\text{N}}{{\text{a}}^ + } + {{\text{e}}^ - } \to {\text{Na(l)}}\);
Award [1 max] for correct equations at incorrect electrodes.
Ignore state symbols.
Accept e instead of \({{\text{e}}^ - }\).
Penalize use of equilibrium signs once only.
Positive electrode (anode):
bromine/\({\text{B}}{{\text{r}}_{\text{2}}}\);
Negative electrode (cathode):
hydrogen/\({{\text{H}}_{\text{2}}}\);
Allow sodium hydroxide/NaOH/hydroxide/\(O{H^ - }\) formation.
bromine/\({\text{B}}{{\text{r}}_{\text{2}}}\);
Do not accept bromide/\(B{r^ - }\).
potential of reduction half-reaction under standard conditions measured relative to standard hydrogen electrode/SHE/potential under standard conditions relative to standard hydrogen electrode/SHE;
Instead of standard state allow either solute concentration of \(1 mol\,d{m^{ - {\text{3}}}}\) or
\(100 kPa/1.00 \times 1{0^5} Pa\) for gases.
Allow 1 bar for \(100 kPa/1.00 \times 1{0^5} Pa\).
Allow 1 atm.
Allow voltage instead of potential.
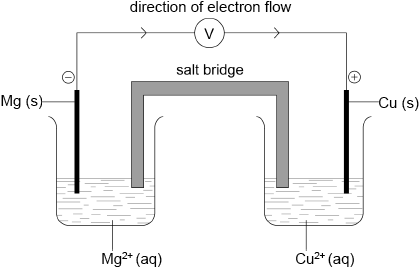
correct diagram including (voltmeter), 4 correct species (state symbols not required) and connecting wires;
No credit if wires to electrodes immersed in the solutions.
Accept ammeter/meter/lamp instead of voltmeter.
labelled salt bridge;
Accept an appropriate salt (name or formula) instead of salt bridge (eg, potassium nitrate).
correctly labelled electrodes as +/cathode and −/anode;
flow of electrons from Mg to Cu in external circuit;
Random uncertainty: (±) 0.01 (V);
Significant figures: 3;
repeat readings and take an average / use more precise equipment;
\({\text{atomization of chlorine}} = \frac{1}{2}{\text{ bond enthalpy / }}\frac{1}{2}{\text{ 243 / 121.5 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
correct values for ionization Na (\( + {\text{496 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)) and electron affinity Cl (\( - {\text{349 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\))
and lattice enthalpy of NaCl (\( + {\text{790 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{ /}} + {\text{769 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\));
Born-Haber energy cycle;
Accept lines or arrows in energy cycle.
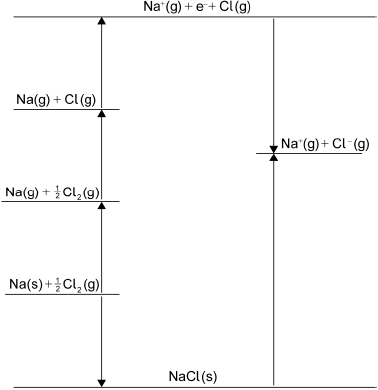
\(\Delta H_{\text{f}}^\Theta {\text{(NaCl(s))}} = - {\text{413.5 /}} - {\text{413 /}} - {\text{414 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept \( - 392.5 / - 392 / - 393 if + 769\) used for lattice enthalpy.
Award [4] for correct final answer.
Examiners report
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.
Candidates found it difficult to write the equation in (a) and the mechanisms in (b) (i) ranged from really good to no understanding. Many opined the production of \( \bullet {\text{H}}\) in the first propagation step. A significant number of candidates suggested a mechanism involving ions despite free radical begin stated in the stem. Most were able to give methanol in (ii). Few scored full marks for (c); the answer needed to be thought through carefully. In (d) the electrodes were often reversed or the equations unbalanced. Few understood the significance of the water present in the answers to (ii). A high percentage of candidates gave the correct answer to (e) but (f) was poorly answered. Either the standard hydrogen electrode or standard conditions were omitted in (i) and the standard of diagrams in (ii) was very poor indeed. Little care seemed to have been taken over their presentation; it was not clear what, if anything, was in the beakers and electrode connections were shown actually in the solutions. In (iii) some did not notice that the voltmeter was digital but most gave the number of significant figures correctly. In (iv) many suggested repeated readings but few stated that an average omitted must be taken. In (g), those who didn’t draw out the cycle tended to get the answer wrong. Examiners cannot give part marks if they cannot work out what is being done. There was one mark for a correct Born-Haber cycle. Very few gained the mark for dividing the chlorine value by 2.

