| Date | May 2018 | Marks available | 1 | Reference code | 18M.1.sl.TZ2.14 |
| Level | SL | Paper | 1 | Time zone | TZ2 |
| Command term | Derive | Question number | 14 | Adapted from | N/A |
Question
What is the enthalpy change of combustion of urea, (NH2)2CO, in kJ mol−1?
2(NH2)2CO(s) + 3O2(g) → 2CO2(g) + 2N2(g) + 4H2O(l)
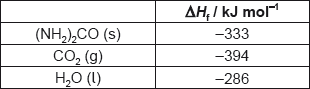
A. 2 × (−333) −2 × (−394) −4 × (−286)
B. \(\frac{1}{2}\)[2 × (−394) + 4 × (−286) −2 × (−333)]
C. 2 × (−394) + 4 × (−286) −2 × (−333)
D. \(\frac{1}{2}\)[2 × (−333) −2 × (−394) −4 × (−286)]
Markscheme
B
Examiners report
[N/A]
Syllabus sections
Show 54 related questions
- 18M.2.hl.TZ2.5b.ii: Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
- 18M.2.hl.TZ2.5b.i: Outline why no value is listed for H2(g).
- 18M.2.sl.TZ2.4b.ii: Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
- 18M.2.sl.TZ2.4b.i: Outline why no value is listed for H2(g).
- 18M.2.sl.TZ1.3b.ii: Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf...
- 18M.1.sl.TZ1.14: What is the enthalpy of combustion of butane in kJ mol−1? 2C4H10(g) + 13O2(g) → 8CO2(g) +...
- 18M.2.hl.TZ1.3c.ii: Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf...
- 17N.1.sl.TZ0.14: The enthalpy changes for two reactions are given. Br2 (l) + F2 (g) → 2BrF (g) ΔH = x...
- 17N.2.hl.TZ0.5a: Calculate the standard enthalpy change for this reaction using the following data.
- 17M.2.hl.TZ2.6b: The overall equation for monochlorination of methane is: CH4(g) + Cl2(g) → CH3Cl(g) +...
- 17M.2.sl.TZ2.8a.i: Calculate the enthalpy change, in kJ, for the spray reaction, using the data...
- 17M.1.sl.TZ2.14: Why is the value of the enthalpy change of this reaction calculated from bond enthalpy data...
- 17M.2.sl.TZ1.4g: The standard enthalpy of formation of N2H4(l) is +50.6 kJ\(\,\)mol−1. Calculate the...
- 17M.1.sl.TZ1.14: Which expression gives the enthalpy change, ΔH, for the thermal decomposition of calcium...
- 16N.1.sl.TZ0.13: Hydrazine reacts with oxygen. N2H4(l) + O2(g) → N2(g) + 2H2O(l) ΔHθ = -623 kJ What is...
- 16M.2.hl.TZ0.2a: (i) Deduce the equilibrium constant expression, Kc, for this reaction. (ii) At exactly 600°C...
- 16M.1.hl.TZ0.16: The equation for the formation of ethyne...
- 16M.1.sl.TZ0.14: What is the enthalpy of formation of ethyne, in...
- 15M.2.hl.TZ2.3a: Calculate the standard enthalpy change, \(\Delta {H^\Theta }\), in...
- 15M.1.sl.TZ2.15: When four moles of aluminium and four moles of iron combine with oxygen to form their oxides,...
- 14M.2.hl.TZ1.1a: (i) Calculate the amount, in mol, of anhydrous magnesium sulfate. (ii) ...
- 14M.2.hl.TZ1.1b: (i) Determine the enthalpy change, \(\Delta H\), in...
- 14M.2.hl.TZ1.1c: Another group of students experimentally determined an enthalpy of hydration of...
- 14M.1.sl.TZ1.15: The enthalpy changes of three reactions are given below. ...
- 14M.2.sl.TZ1.1b: (i) Calculate the amount, in mol, of anhydrous magnesium sulfate. (ii) Calculate...
- 14M.2.sl.TZ1.1c: (i) Determine the enthalpy change, \(\Delta H\), in...
- 14M.2.sl.TZ1.1d: Another group of students experimentally determined an enthalpy of hydration of...
- 14N.2.hl.TZ0.2b: (i) Define the term standard enthalpy change of formation. (ii) ...
- 14N.2.hl.TZ0.2c: Comment on which of the values obtained in (a) and (b)(ii) is more accurate, giving a reason.
- 14N.2.hl.TZ0.11e: Hydrochloric acid neutralizes sodium hydroxide, forming sodium chloride and...
- 14N.1.sl.TZ0.15: Consider the following equations. ...
- 14N.2.sl.TZ0.7e: (i) Define the term standard enthalpy change of reaction, \(\Delta {H^\Theta...
- 13N.1.hl.TZ0.16: Consider the following two equations. ...
- 13N.1.sl.TZ0.16: Consider the following two...
- 13M.2.hl.TZ1.8b.i: Calculate the standard enthalpy change of this reaction, using the values of enthalpy of...
- 13M.2.sl.TZ1.7b: Methanol can be produced according to the following...
- 13M.1.hl.TZ2.15: Enthalpy changes of reaction are provided for the following...
- 13M.2.sl.TZ2.2a: The standard enthalpy change of three combustion reactions are given...
- 12N.1.sl.TZ0.16: Using the equations...
- 10N.1.hl.TZ0.15: Consider the equations...
- 10N.1.hl.TZ0.16: Given the enthalpy change for the reaction...
- 09N.2.hl.TZ0.7b.i: The standard enthalpy change of three combustion reactions is given below in...
- 09N.2.sl.TZ0.3: The standard enthalpy change of three combustion reactions is given below in...
- 10M.1.sl.TZ2.15: The standard enthalpy changes for the combustion of carbon and carbon monoxide are shown...
- 09M.2.sl.TZ1.2b: Marit arranged the values she found in Table 12 into an energy cycle. Calculate the value...
- 11M.1.hl.TZ1.17: Consider the two reactions involving iron and...
- 11M.1.sl.TZ1.16: Consider the following...
- 11M.1.sl.TZ2.16: Consider the following...
- 11M.2.sl.TZ2.1b.iii: Using the values obtained for \(\Delta {H_1}\) in (a) (iv) and \(\Delta {H_2}\) in (b) (ii),...
- 12M.1.sl.TZ2.16: Consider the...
- 12M.1.sl.TZ2.15: A simple calorimeter was set up to determine the enthalpy change occurring when one mole of...
- 12M.2.sl.TZ2.6e: When 5.35 g ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(s)}}\), is added...
- 12M.2.sl.TZ2.6d: (i) Sketch and label an enthalpy level diagram for this reaction. (ii) Deduce...
- 11N.1.sl.TZ0.16: Consider the following enthalpy of combustion...

