| Date | May 2009 | Marks available | 1 | Reference code | 09M.2.sl.TZ1.2 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Calculate | Question number | 2 | Adapted from | N/A |
Question
Two students were asked to use information from the Data Booklet to calculate a value for the enthalpy of hydrogenation of ethene to form ethane.
\[{{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_6}{\text{(g)}}\]
John used the average bond enthalpies from Table 10. Marit used the values of enthalpies of combustion from Table 12.
John then decided to determine the enthalpy of hydrogenation of cyclohexene to produce cyclohexane.
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{10}}}}{\text{(l)}} + {{\text{H}}_{\text{2}}}{\text{(g)}} \to {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{\text{(l)}}\]
Calculate the value for the enthalpy of hydrogenation of ethene obtained using the average bond enthalpies given in Table 10.
Marit arranged the values she found in Table 12 into an energy cycle.
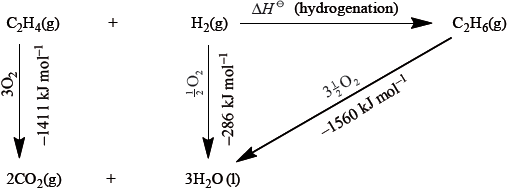
Calculate the value for the enthalpy of hydrogenation of ethene from the energy cycle.
Suggest one reason why John’s answer is slightly less accurate than Marit’s answer.
Use the average bond enthalpies to deduce a value for the enthalpy of hydrogenation of cyclohexene.
The percentage difference between these two methods (average bond enthalpies and enthalpies of combustion) is greater for cyclohexene than it was for ethene. John’s hypothesis was that it would be the same. Determine why the use of average bond enthalpies is less accurate for the cyclohexene equation shown above, than it was for ethene. Deduce what extra information is needed to provide a more accurate answer.
Markscheme
energy required = C=C + H–H/612 + 436 and
energy released = C–C + 2(C–H)/347 + 2(413) /
energy required = C=C + H–H + 4(C–H)/612 + 436 + 4(413) and
energy released = C–C + 6(C–H)/347 + 6(413);
\(\Delta H = - 1411 + ( - 286) - ( - 1560) = - 137{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
the actual values for the specific bonds may be different to the average values / the combustion values referred to the specific compounds / OWTTE;
\( - {\text{125 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
average bond enthalpies do not apply to the liquid state / OWTTE;
the enthalpy of vaporization/condensation of cyclohexene and cyclohexane / OWTTE;
Examiners report
Candidates struggled with Part (a). The most common errors were those of calculation, incorrect identification of the bonds involved and a final answer with the opposite sign and missing units.
In (b) many candidates found it difficult to use Hess’ Law with the cycle presented in this form, a good proportion not recognising that this was, indeed, a Hess’ Law calculation.
In Part (c) many of the candidates simply repeated the question, giving no reason or explanation for the likely difference in accuracy.
Many candidates repeated the calculation from (a) in (d)(i) instead of realising that the question asked for a deduction rather than another calculation. Credit was given if the same (even if incorrect) answer was obtained as in part (a).
In (d)(ii) very few candidates seemed to notice that this process involved substances in the liquid state hence the need for enthalpies of vaporization/condensation. It was commonly thought that the position of the double bond in the cyclohexene ring would make a significant difference.

