| Date | May 2011 | Marks available | 3 | Reference code | 11M.2.hl.TZ1.7 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Calculate | Question number | 7 | Adapted from | N/A |
Question
In an experiment conducted at 25.0 °C, the initial concentration of propanoic acid and methanol were \({\text{1.6 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and \({\text{2.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) respectively. Once equilibrium was established, a sample of the mixture was removed and analysed. It was found to contain \({\text{0.80 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) of compound X.
Two compounds, A and D, each have the formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Cl}}\).
Compound A is reacted with dilute aqueous sodium hydroxide to produce compound B with a formula of \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\). Compound B is then oxidized with acidified potassium
manganate(VII) to produce compound C with a formula of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). Compound C resists further oxidation by acidified potassium manganate(VII).
Compound D is reacted with dilute aqueous sodium hydroxide to produce compound E with a formula of \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\). Compound E does not react with acidified potassium manganate(VII).
Deduce the structural formulas for compounds A, B, C, D and E.
A:
B:
C:
D:
E:
Deduce an equation for the reaction between propanoic acid and methanol. Identify the catalyst and state the name of the organic compound, X, formed.
Calculate the concentrations of the other three species present at equilibrium.
State the equilibrium constant expression, \({K_{\text{c}}}\), and calculate the equilibrium constant for this reaction at 25.0 °C.
2-chloro-3-methylbutane reacts with sodium hydroxide via an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Explain the mechanism by using curly arrows to represent the movement of electron pairs.
Explain why the hydroxide ion is a better nucleophile than water.
1-chlorobutane can be converted to a pentylamine via a two stage process. Deduce equations for each step of this conversion including any catalyst required and name the organic product produced at each stage.
Markscheme
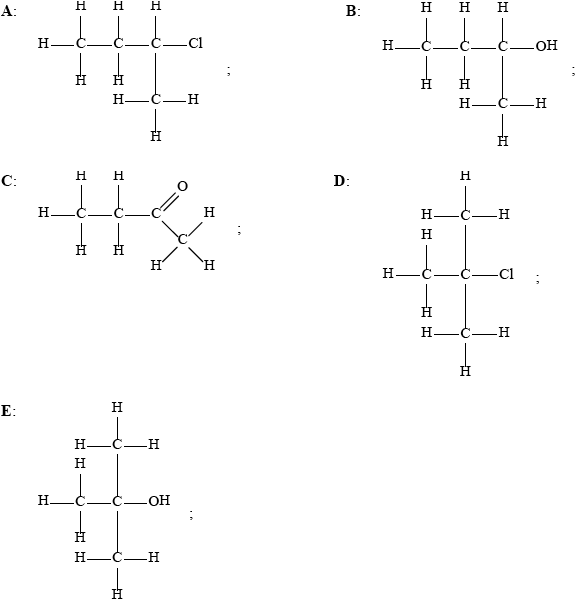
Accept condensed formulas.
Award [1 max] if A and D are other way round (and nothing else correct).
Award [2 max] if A and D are other way round but one substitution product B or E is correct based on initial choice of A and D.
Award [3 max] if A and D are other way round but both substitution products B and E are correct based on initial choice of A and D.
M2 (for B) and M5 (for E) may also be scored for substitution product if primary chloroalkane used.
Penalize missing hydrogens once only in Q.7.
\[{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{COOH}} + {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOC}}{{\text{H}}_{\text{3}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
[1] for reactants and [1] for products.
(concentrated) sulfuric acid/\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\);
Do not accept just \({H^ + }\) or acid.
methyl propanoate;
[CH3CH2COOH]:
\((1.6 - 0.80 = ){\text{ }}0.8{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
[CH3OH]:
\((2.0 - 0.80 = ){\text{ }}1.2{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
[H2O]:
\({\text{0.80 (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\(({K_{\text{c}}} = )\frac{{{\text{[C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOC}}{{\text{H}}_{\text{3}}}{\text{][}}{{\text{H}}_{\text{2}}}{\text{O]}}}}{{{\text{[C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH][C}}{{\text{H}}_{\text{3}}}{\text{OH]}}}}\);
\(\left( {{K_{\text{c}}} = \frac{{[{{(0.80)}^2}]}}{{\left[ {(1.2 \times 0.8)} \right]}} = } \right){\text{ }}0.7\);
Allow 0.67.
Award [1 max] for 0.83.
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to C;
Do not allow curly arrow originating on H in \(H{O^ - }\).
curly arrow showing Cl leaving;
Accept curly arrow either going from bond between C and Cl to Cl in 2-chloro-3-methylbutane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Cl are not at 180° to each other.
Do not award M3 if OH ---- C bond is represented.
formation of organic product 3-methylbutan-2-ol and \({\text{C}}{{\text{l}}^ - }\);
\({\text{O}}{{\text{H}}^ - }\) has a negative charge/higher electron density;
greater attraction to the carbon atom (with the partial positive charge) / OWTTE;
Do not allow just greater attraction.
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Cl}} + {\text{KCN}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CN}} + {\text{KCl}}\);
Accept \(C{N^ - }\) for KCN and \(C{l^ - }\) for KCl.
pentanenitrile;
Allow 1-cyanobutane.
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{CN}} + {\text{2}}{{\text{H}}_2} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{N}}{{\text{H}}_2}\);
pentan-1-amine / 1-aminopentane / 1-pentylamine / 1-pentanamine;
Catalyst: nickel/Ni / palladium/Pd / platinum/Pt;
Penalise missing hydrogen once only in Q.7.
Examiners report
This was the least popular question in Section B. Most candidates either scored all five marks in (a) or just one.
(b) was usually well done, though it was disappointing that more candidates did not use the equilibrium sign.
In (c), a significant number of candidates omitted water from the equilibrium calculations.
In (c), a significant number of candidates omitted water from the equilibrium calculations.
The organic reaction mechanism in (d) (i) was very poorly presented. Many even tried drawing curly arrows from NaOH as an attacking species. The majority could identify the product of the reaction but a mechanism was far beyond them. Transition states were poor or missing completely.
In (ii) although many knew that \({\text{O}}{{\text{H}}^ - }\) has a negative charge, few linked this to the greater attraction to the carbon atom.
In (iii) very few candidates did well here and the name of pentan-1-amine was rarely given. Other mistakes included incorrect catalysts. Further common mistakes included some candidates not including all the hydrogens in the structural formulas. In general for this part there was very poor knowledge of organic synthesis amongst candidates. Very few had a good “stab” at this question. The fact that pentylamine was mentioned in the question initially meant that very few candidates accessed the last mark for the name of the product.

