Question 1


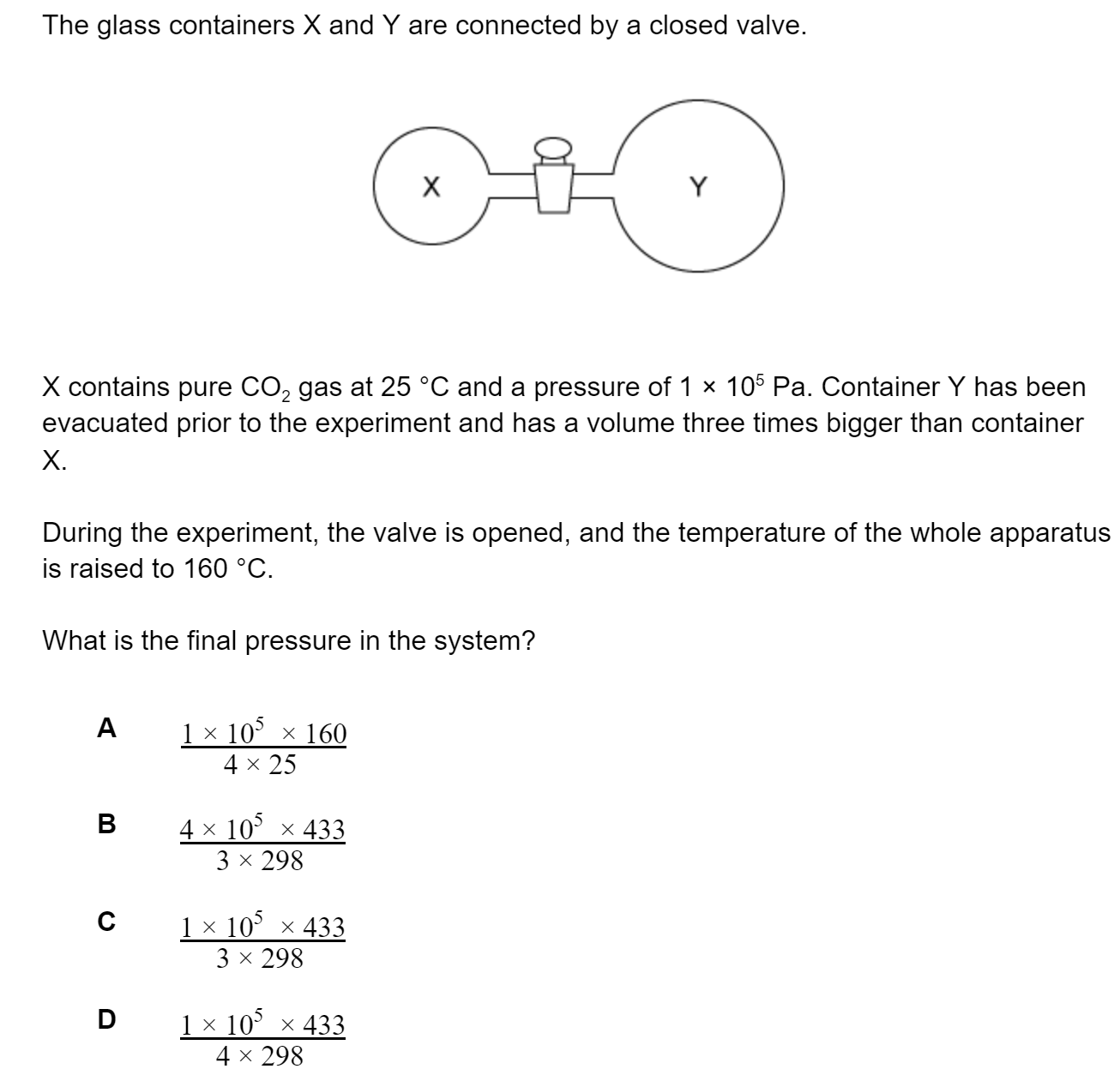
Successive ionisation energies for an element, Y, are shown in the table below.
| Electrons removed |
1st |
2nd |
3rd |
4th |
5th |
| Ionisation energy / kJ mol-1 |
736 |
1450 |
7740 |
10500 |
13600 |
What is the most likely formula for the ion of Y?
Y+
Y2+
Y3+
Y4+

Use a periodic table to deduce the correct information about the element tin, Sn (Z = 50)
| Number of occupied main energy levels |
Number of electrons in the highest main energy level |
|
|
A |
4 |
4 |
|
B |
4 |
14 |
|
C |
5 |
4 |
|
D |
5 |
14 |
Which complex is likely to be colourless?
[Zn(H2O)6]Cl2
[NH4]2[Fe(H2O)6][SO4]2
K3[Co(CN)6]
[Ni(NH3)6][BF4]2
Which type of bonding can be described as ‘the electrostatic attraction between positive nuclei and electrons and occurs by the sharing of electrons’?
Hydrogen bonding
Ionic bonding
Metallic bonding
Covalent bonding
Which of the following are properties of transition metals?
I and II only
I and III only
II and III only
I, II and III
Which is a characteristic property of sodium oxide?
It turns moist litmus paper blue
It turns moist litmus paper red
When it dissolves in distilled water it forms a solution with pH less than 7
It reacts with magnesium metal
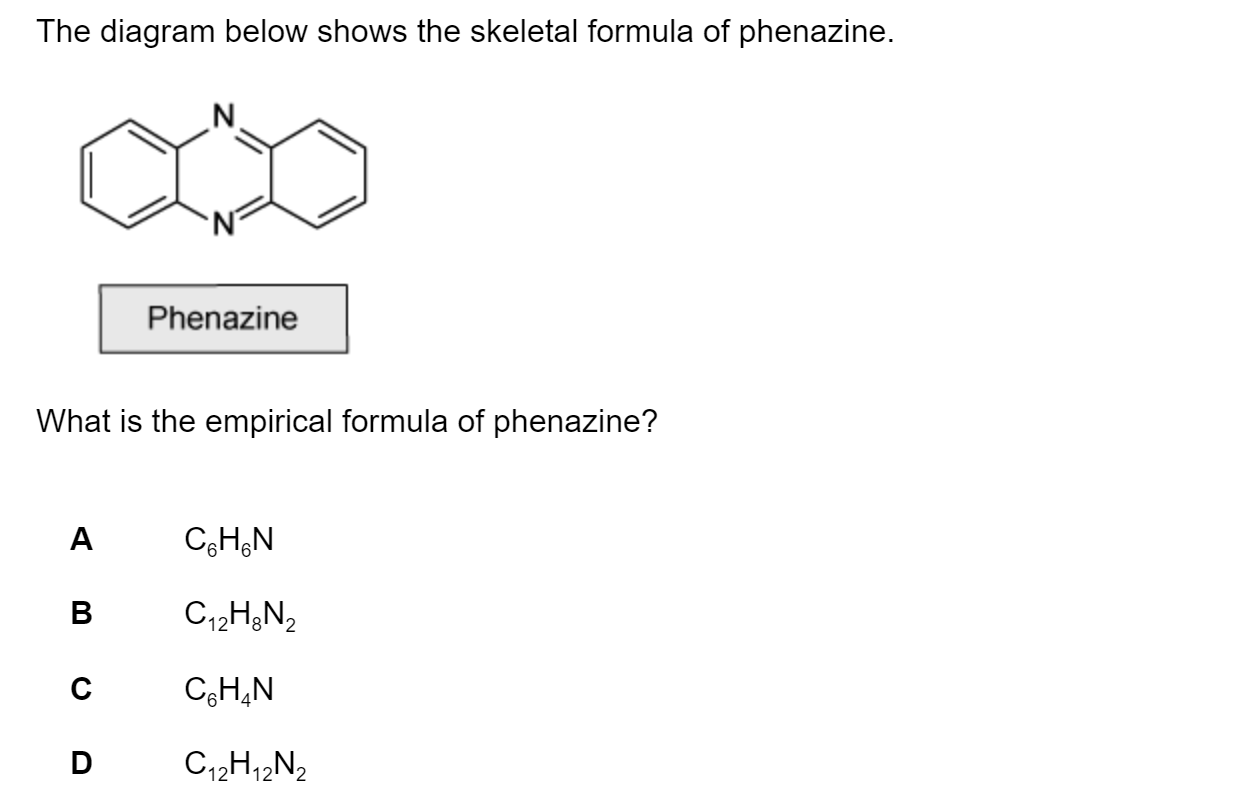
Sulfur forms the compound S4N4 with nitrogen, the structure is shown below.
Which of the following statements about S4N4 is correct?
The sulfur atom marked x has two lone pairs
The sulfur atom marked y has two lone pairs
Each N atom has two lone pairs
The N-S-N bond angle will be larger than S=N=S bond angle
Which combination describes the PH4+ ion?
|
|
Molecular geometry |
Central atom hybridisation |
|
A |
Tetrahedral |
sp3 |
|
B |
Square planar |
sp3 |
|
C |
Tetrahedral |
sp2 |
|
D |
Square planar |
sp2 |
Which enthalpy changes correctly describe the following reactions?
Reaction 1: HNO3 (aq) + NH3 (aq) → NH4NO3 (aq)
Reaction 2: CuCO3 (s) → CuO (s) + CO2 (g)
Reaction 3: S (s) + O2 (g) → SO2 (g)
|
ΔHӨc |
ΔHӨf |
ΔHӨneut |
ΔHӨr |
|
|
A |
2 |
2 |
1 |
3 |
|
B |
3 |
2 |
1 |
2 |
|
C |
3 |
3 |
1 |
2 |
|
D |
2 |
3 |
1 |
3 |
Bond energy calculations show the enthalpy of combustion for propene to be -1572.0 kJ mol-1.
|
Compound |
C3H6 (g) |
CO2 (g) |
H2O (l) |
H2O (g) |
|
ΔHӨf / kJ mol-1 |
+20.0 |
-393.5 |
-285.8 |
-241.8 |
Using the enthalpy of formation data, which calculation correctly shows the percentage error between propene’s enthalpy of combustion values obtained from bond energy calculations and Hess’s Law calculations, assuming the bond energy calculation value is correct?
The ΔGϴf values for the following substances are shown.
|
Substance |
ΔGϴf (kJ mol-1) |
|
NH3 (g) |
-16.4 |
|
O2 (g) |
0 |
|
H2O (g) |
-228.6 |
|
NO (g) |
87.6 |
Which of the following is the correct calculation to determine ΔGϴ?
4NH3 (g) + 5O2 (g) ⇌ 6H2O (g) + 4NO (g)
(-228.6 + 87.6) + (-16.4)
(-16.4 x 4) - [(-228.6 x 6) + (87.6 x 4)]
[-228.6 + (87.6 x 4)] - (-16.4 x 4)
[(-228.6 x 6) + (87.6 x 4)] - (-16.4 x 4)
Which statements are correct for ionic compounds?
I and II only
I and III only
II and III only
I, II and III

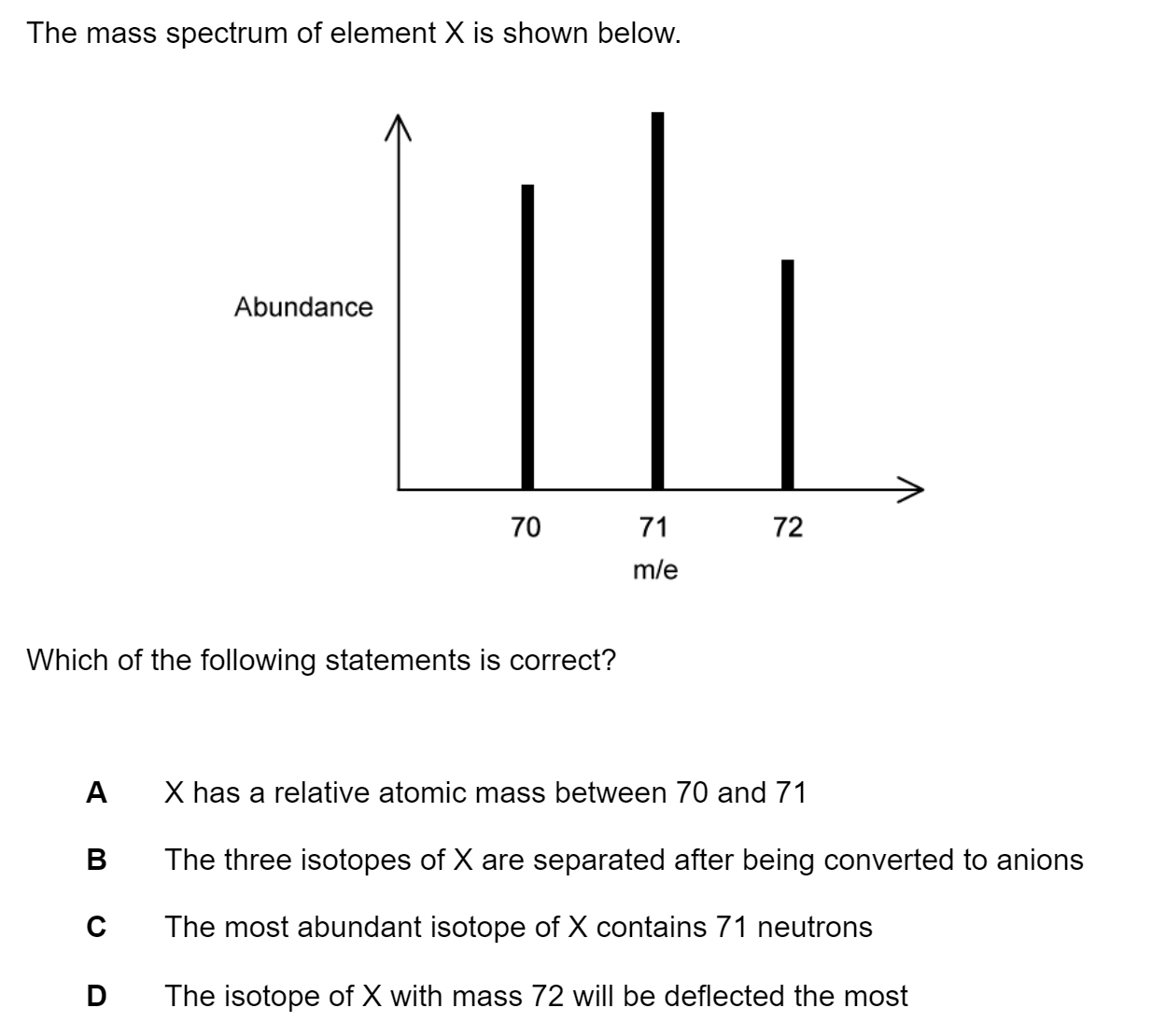
A student experimentally determined the rate expression for the reaction between iodine and propanone to be:
Rate = k [H+] [C3H6O]
Which graph is consistent with this information?

The following information was obtained for the rate constant, k, for a reaction at 25 OC
|
A |
Ea |
R |
|
2.57 × 109 s–1 |
96.2 kJ mol–1 |
8.31 J K–1 mol–1 |
Which expression correctly represents how to calculate the rate constant, k?
k = (2.57 x 109) x e(-96.2 / 8.31×25)
k = (2.57 x 109) x e(-96.2 / 8.31×298)
k = (2.57 x 109) x e(-96200 / 8.31×25)
k = (2.57 x 109) x e(-96200 / 8.31×298)
Consider the following reversible reaction:
3O2 (g) ⇌ 2O3 (g)
What is the value of Kc when the equilibrium concentrations are [O2] = 4.0 mol dm-3 and [O3] = 4.0 mol dm-3 ?
0.25
4
16
64
Which of the following equations is used when calculating the temperature, in Kelvin, at which a reaction becomes feasible if ΔHΘ = x and ΔSΘ = y.
T =
T = x y
T = x + y
T =
In the Brønsted–Lowry theory of acids and bases, the difference between a conjugate acid and its conjugate base is the presence of which of the following?
a positive charge
a pair of electrons
a proton
a hydrogen atom
How many different types of ions can be found in acid rain, assuming it contains a mixture of sulfuric, sulfurous, nitric and nitrous acids?
4
5
6
7
Which of the following statements are correct for a titration between 0.10 mol dm-3 propanoic acid and 0.10 mol dm-3 potassium hydroxide?
The equivalence point will be at pH 7
The salt formed will hydrolyse to form an acidic salt
The salt formed will be CH3COOK
At half equivalence point [CH3CH2COOH (aq)] = [CH3CH2COO- (aq)]
Which of the following statements is correct?
As temperature increases, the pH value of pure water decreases
As temperature decreases, the pH value of pure water decreases
The pH of water is unaffected by temperature
Pure water is not neutral
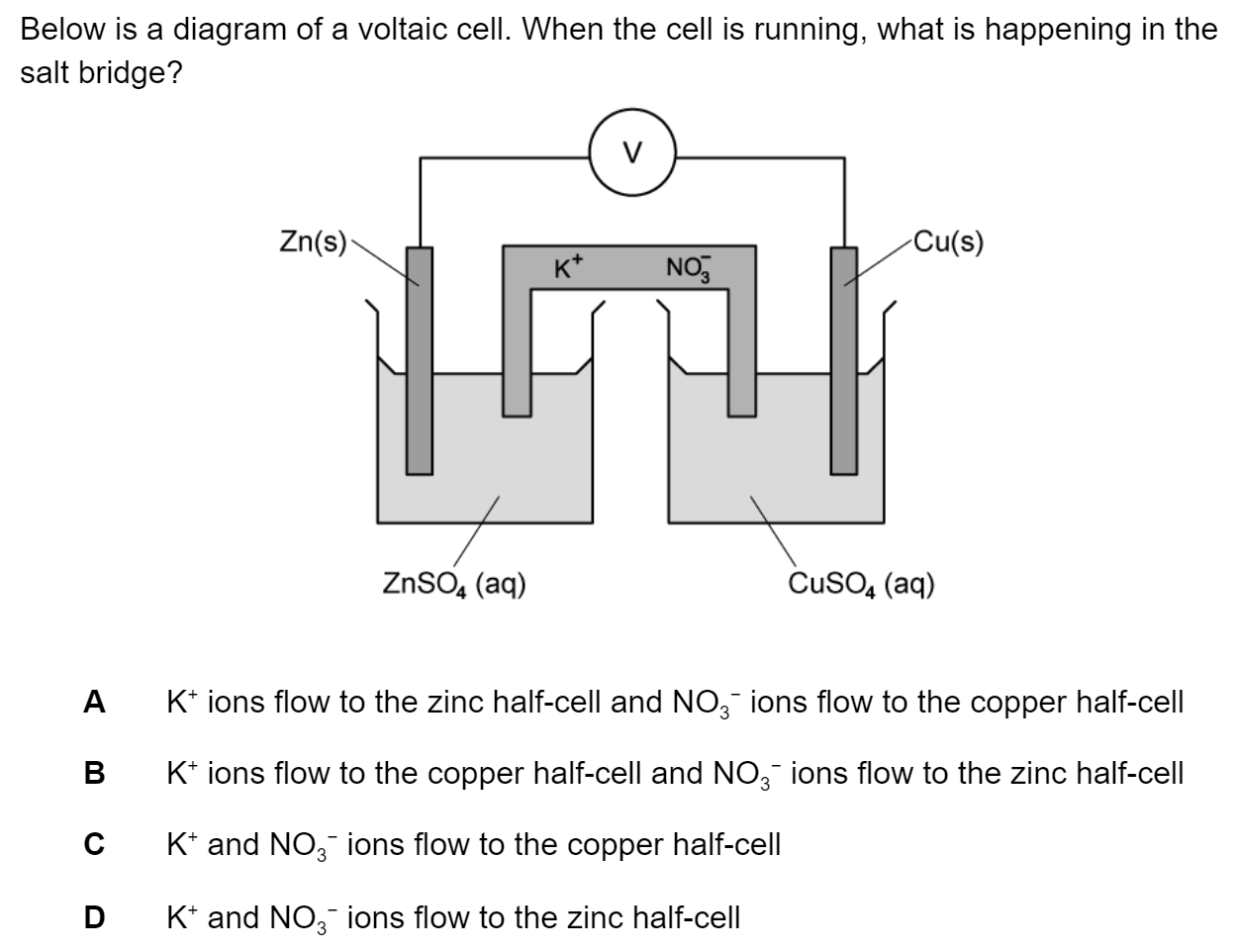
Which of the following reactions could take place at the positive electrode (cathode) in a voltaic cell?
I and II only
I and III only
II and III only
I, II and III
Consider these standard electrode potentials.
Fe2+ (aq) + 2e- Fe (s) EΘ = -0.45 V
Cu2+ (aq) + 2e- Cu (s) EΘ = +0.15 V
Which is the correct working to determine EΘcell?
EΘcell = 0.15 - (-0.45)
EΘcell = 0.15 + (-0.45)
EΘcell = (-0.45) - 0.15
EΘcell = 0.15 x (-0.45)
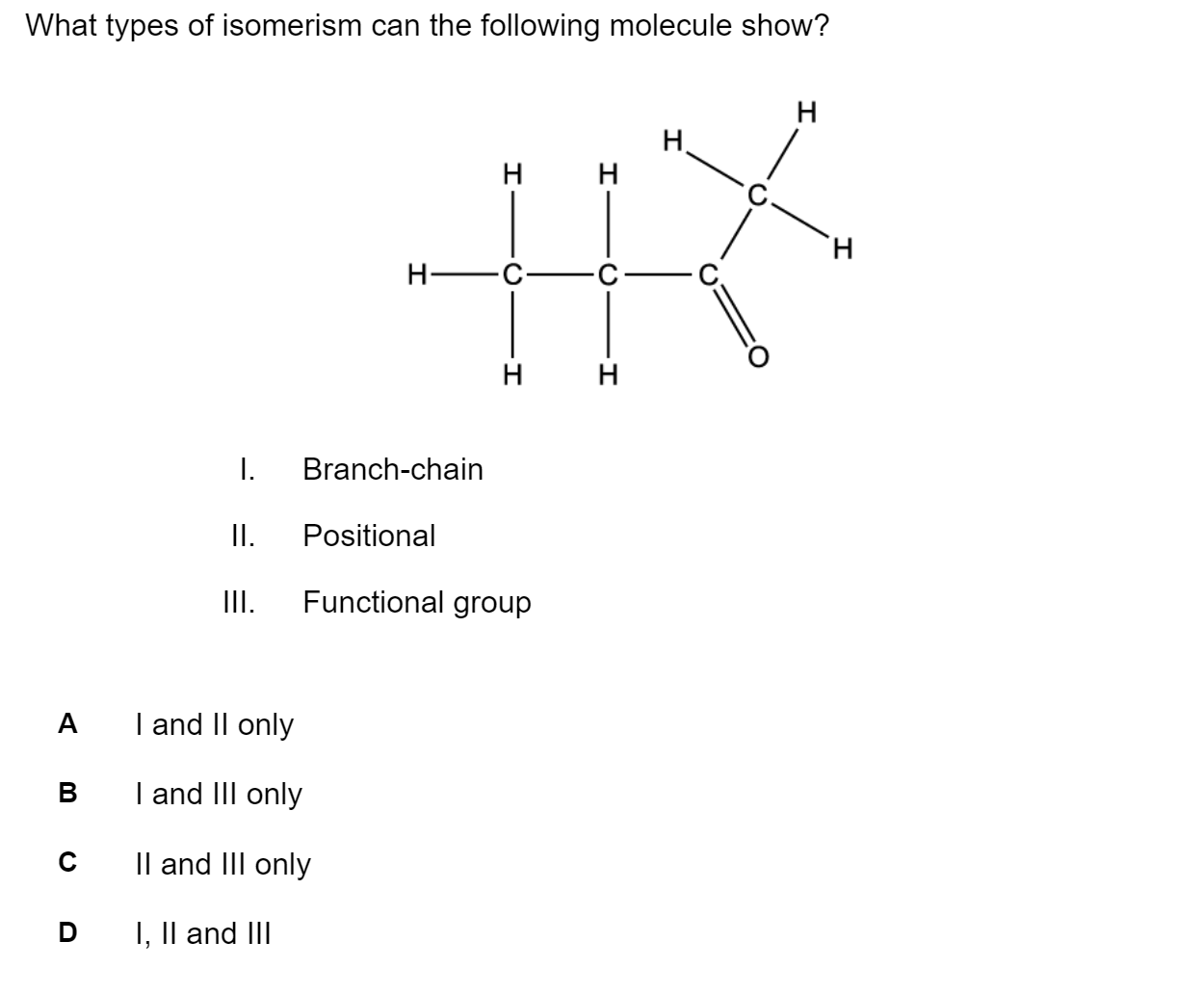
Which factors affect the rate of nucleophilic substitution in halogenoalkanes?
I and II only
I and III only
II and III only
I, II and III
An alkene, X, undergoes electrophilic addition with hydrogen bromide to form a halogenoalkane, Y, as the minor product.
The halogenoalkane, Y , can react with sodium hydroxide in aqueous conditions to form butan-1-ol.
What is the correct identity of the alkene, X?
2-methylpropene
But-1-ene
But-2-ene
Pent-1-ene
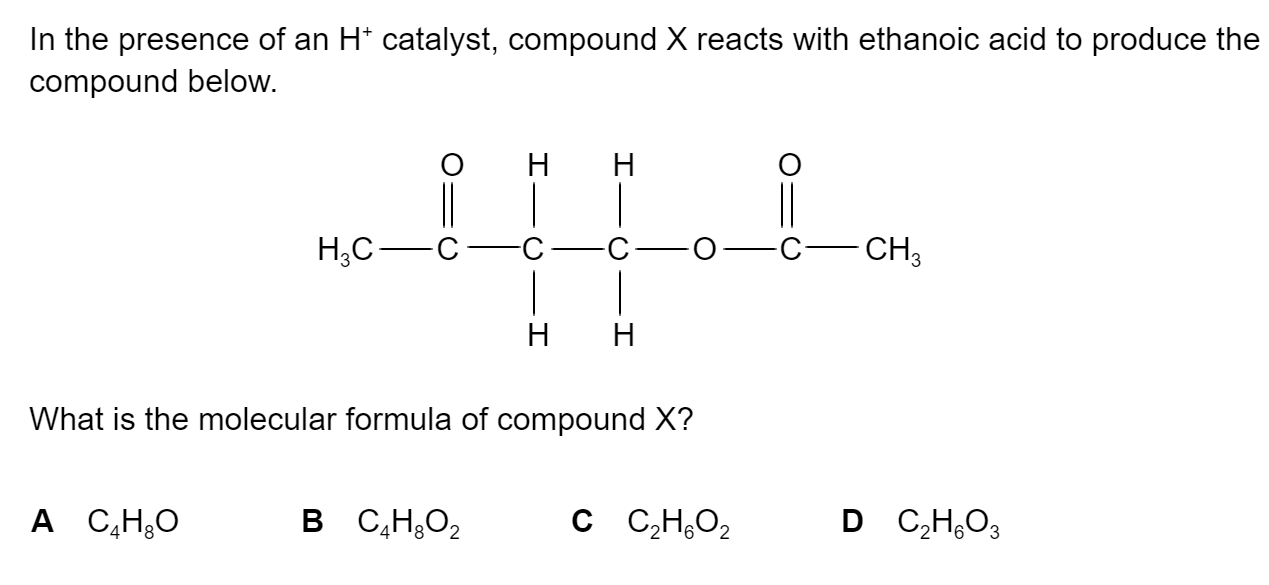
What is this molecule called?
Z-2-bromo-1-chloro-2-fluoroethene
E-2-bromo-1-chloro-2-fluoroethene
Z-1-bromo-2-chloro-1-fluoroethene
E-1-bromo-2-chloro-1-fluoroethene
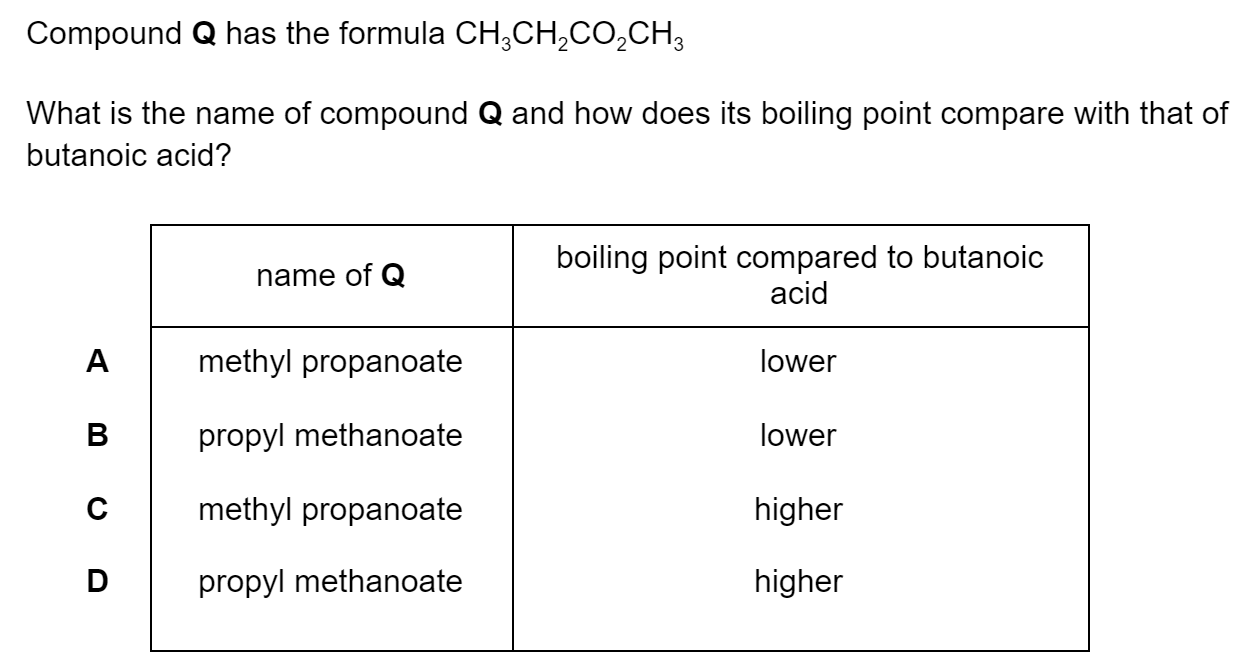
The synthesis of 2-propyl propanoate can be carried out in two steps:
I
CH3COCH3 → CH3CH(OH)CH3
II
CH3CH(OH)CH3 → C2H5COOCH(CH3)2
What are the reagents needed in I and II?
|
|
I |
II |
|
A |
potassium dichromate(VI) |
sulfuric acid, propanoic acid |
|
B |
sodium borohydride |
sulfuric acid, propanoic acid |
|
C |
sodium borohydride |
sulfuric acid, ethanoic acid |
|
D |
potassium dichromate(VI) |
sulfuric acid, ethanoic acid |

The 1H NMR spectrum of CH3CHCl2 shows two signals.
What is the correct assignment of splitting patterns for these signals?
| CH3 group | CH group | |
| A | doublet | quartet |
| B | quartet | doublet |
| C | singlet | singlet |
| D | triplet | singlet |
Which row correctly describes the splitting pattern observed on the 1H NMR spectrum for each labelled hydrogen?
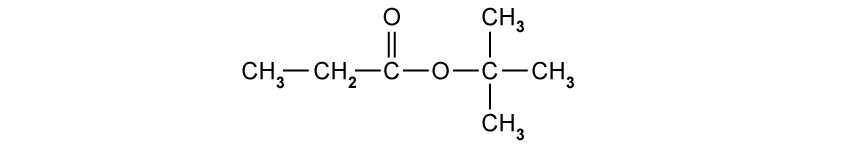
One doublet and four triplets
One triplet, one quartet and one singlet
One triplet, one doublet and three singlets
One triplet, one quartet and three singlets