| Date | May 2018 | Marks available | 1 | Reference code | 18M.2.sl.TZ2.3 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Outline | Question number | 3 | Adapted from | N/A |
Question
The emission spectrum of an element can be used to identify it.
Elements show trends in their physical properties across the periodic table.
Draw the first four energy levels of a hydrogen atom on the axis, labelling n = 1, 2, 3 and 4.
Draw the lines, on your diagram, that represent the electron transitions to n = 2 in the emission spectrum.
Outline why atomic radius decreases across period 3, sodium to chlorine.
Outline why the ionic radius of K+ is smaller than that of Cl−.
Copper is widely used as an electrical conductor.
Draw arrows in the boxes to represent the electronic configuration of copper in the 4s and 3d orbitals.
Impure copper can be purified by electrolysis. In the electrolytic cell, impure copper is the anode (positive electrode), pure copper is the cathode (negative electrode) and the electrolyte is copper(II) sulfate solution.
Formulate the half-equation at each electrode.
Outline where and in which direction the electrons flow during electrolysis.
Markscheme
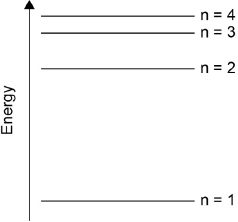
4 levels showing convergence at higher energy
[1 mark]
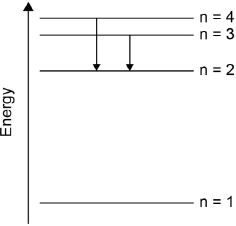
arrows (pointing down) from n = 3 to n = 2 AND n = 4 to n = 2
[1 mark]
same number of shells/«outer» energy level/shielding AND nuclear charge/number of protons/Zeff increases «causing a stronger pull on the outer electrons»
[1 mark]
K+ 19 protons AND Cl– 17 protons
OR
K+ has «two» more protons
same number of electrons/isoelectronic «thus pulled closer together»
[2 marks]
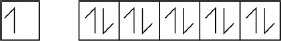
[1 mark]
Anode (positive electrode):
Cu(s) → Cu2+(aq) + 2e–
Cathode (negative electrode):
Cu2+(aq) + 2e– → Cu(s)
Accept Cu(s) – 2e– → Cu2+(aq).
Accept for →
Award [1 max] if the equations are at the wrong electrodes.
[2 marks]
«external» circuit/wire AND from positive/anode to negative/cathode electrode
Accept “through power supply/battery” instead of “circuit”.
[1 mark]

