| Date | November 2013 | Marks available | 1 | Reference code | 13N.2.hl.TZ0.6 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Identify | Question number | 6 | Adapted from | N/A |
Question
In acidic solution, ions containing titanium can react according to the half-equation below.
\({\text{Ti}}{{\text{O}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}\) \({E^\Theta } = - 0.06{\text{ V}}\)
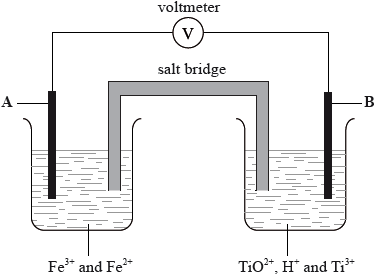
In the diagram below, A and B are inert electrodes and, in the aqueous solutions, all ions have a concentration of \({\text{1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\).
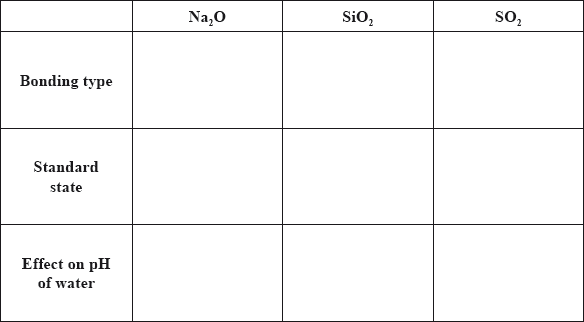
Sodium, silicon and sulfur are elements in period 3 of the periodic table that all form oxides.
Although carbon and silicon both belong to group 4 of the periodic table, carbon dioxide and silicon dioxide are different in many ways.
Define the term standard electrode potential, \({E^\Theta }\).
State the initial and final oxidation numbers of titanium and hence deduce whether it is oxidized or reduced in this change.

Considering the above equilibrium, predict, giving a reason, how adding more acid would affect the strength of the \({\text{Ti}}{{\text{O}}^{2 + }}\) ion as an oxidizing agent.
In the two experiments below, predict whether a reaction would occur and deduce an equation for any reaction that takes place. Refer to Table 14 of the Data Booklet if necessary.
KI(aq) is added to a solution containing \({\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}}\) ions:
Zn (s) is added to a solution containing \({\text{Ti}}{{\text{O}}^{2 + }}{\text{(aq)}}\) and \({{\text{H}}^ + }{\text{(aq)}}\) ions:
Using Table 14 of the Data Booklet, state the balanced half-equation for the reaction that occurs at electrode A and whether it involves oxidation or reduction.
Calculate the cell potential in V.
On the diagram above label with an arrow
• the direction of electron flow in the wire
• the direction in which the positive ions flow in the salt bridge.
Compare the properties of the three oxides by completing the table below.
Sulfur dioxide is a significant contributor to acid deposition. Identify a major, man-made source of this pollutant.
As well as the oxide above, sodium forms a peroxide that contains the peroxide ion, \({\text{O}}_2^{2 - }\). Draw the Lewis (electron dot) structure of the peroxide ion.
Describe the differences in the hybridization of these group 4 elements and the precise nature of the bonds that they form with the oxygen atoms.
Xenon, although a noble gas, forms an oxide, \({\text{Xe}}{{\text{O}}_{\text{2}}}\), that has a structure related to that of \({\text{Si}}{{\text{O}}_{\text{2}}}\). Compare the geometry around the silicon atoms in \({\text{Si}}{{\text{O}}_{\text{2}}}\) with the geometry around the xenon atoms in \({\text{Xe}}{{\text{O}}_{\text{2}}}\), using the valence shell electron pair repulsion (VSEPR) theory.
Markscheme
potential of the half-cell / reduction half-reaction under standard conditions measured relative to standard hydrogen electrode/SHE;
Allow instead of standard conditions, solute concentration of 1 mol dm–3 or 1 bar/1 atm (pressure) for gases.

+ sign must be present. Do not award mark for incorrect notation 4, 4+, 3, 3+ etc.
Do not award M2 if inconsistent with M1.
increases / makes it stronger;
(more \({{\text{H}}^ + }\) would) drive/shift equilibrium to the right/towards products (accepting more electrons);
KI(aq) is added to a solution containing Ti3+(aq) ions:
no reaction;
Zn(s) is added to a solution containing TiO2+(aq) and H+(aq) ions:
\({\text{Zn(s)}} + {\text{2Ti}}{{\text{O}}^{2 + }}{\text{(aq)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{Z}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{2T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\)
correct reactants and products;
balanced equation;
Ignore state symbols.
\({\text{F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {{\text{e}}^ - } \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\);
Ignore state symbols.
Accept equilibrium arrow.
reduction;
Do not apply ECF.
\(( + 0.77 - ( - 0.06)) = ( + )0.83{\text{ (V)}}\);
Do not accept –0.83 V.
wire and salt bridge both have arrows from B to A;
Accept arrows above or below each provided it is obvious which they refer to.
Apply ECF from part (i).
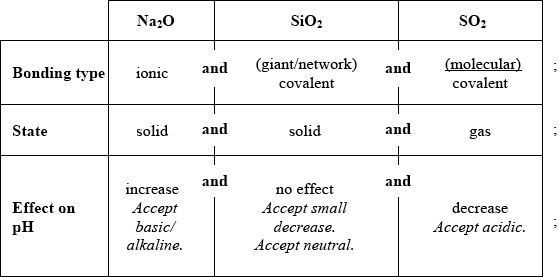
For any parts (properties) where mark not awarded, award [1] for every three correct responses.
(combustion of) coal / diesel;
Accept “burning of fossil fuels”, “industrial processes” or “combustion/car engines”.
Do not accept “Contact process”.

e-pairs correct;
charges in correct positions;
Accept lines, or pairs of dots or crosses, for electron pairs.
Accept 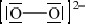 .
.
C is sp hybridized and Si is \({\text{s}}{{\text{p}}^{\text{3}}}\) hybridized;
C–O bond in \({\text{C}}{{\text{O}}_{\text{2}}}\) has one \(\sigma \)-bond and one \(\pi \)-bond;
Si–O bond in \({\text{Si}}{{\text{O}}_{\text{2}}}\) has one \(\sigma \)-bond only;
Award [1 max] for last two marking points for “C–O double bond and Si–O single bond”.
silicon-oxygen bonds will have a tetrahedral distribution;
xenon-oxygen bonds will have a square planar distribution;
xenon dioxide has two non-bonding/lone pairs of electrons;
Award any of the above marks if clearly indicated in suitable diagrams.
Examiners report
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.
The required definition and the effect of acid on the oxidizing power of \({\text{Ti}}{{\text{O}}^{2 + }}\) was often well done, though it proved a challenge for some candidates, and most could interpret the change in terms of oxidation numbers. Very few candidates could use \({E^\Theta }\) values to predict whether a reaction with another half-cell would occur and even less could correctly combine the half-equations to produce a balanced equation for the overall reaction. Relatively few candidates managed to gain full marks for the questions relating to the voltaic cell illustrated, with the different parts appearing to be of approximately equal difficulty. The nature of the period 3 oxides was generally well appreciated, though often the effect on pH was expressed as, for example, “basic” rather than “increases”. In spite of the efficiency of modern plants many considered the contact process to be a major source of sulfur dioxide pollution, rather than combustion of coal and other “high sulfur” fossil fuels. The comparison of the structure of silicon dioxide to those of carbon and xenon dioxides was poorly done, the root cause often being a lack of awareness of the structure of silicon dioxide. Many candidates could however write correct equations for the reaction of silicon tetrachloride with water.

