| Date | November 2014 | Marks available | 4 | Reference code | 14N.3.sl.TZ0.9 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | Outline | Question number | 9 | Adapted from | N/A |
Question
Iron acts as a catalyst in the chemical reactions below.
Reaction I, catalysed by \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\): \({{\text{S}}_{\text{2}}}{\text{O}}_8^{2 - }{\text{(aq)}} + {\text{2}}{{\text{I}}^ - }{\text{(aq)}} \to {\text{2SO}}_4^{2 - }{\text{(aq)}} + {{\text{I}}_{\text{2}}}{\text{(aq)}}\)
Reaction II, catalysed by Fe(s): \({\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} + {{\text{N}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\)
State the type of catalysis occurring in reaction I.
Outline the mechanism by which each catalyst lowers the activation energy in the reactions above, and state a particular disadvantage of each type of catalysis.
Markscheme
homogeneous;
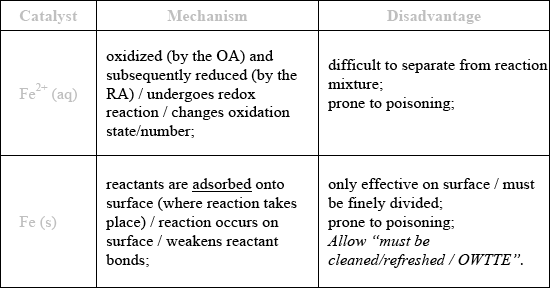
Allow “prone to poisoning” as a disadvantage for either but not both.
Examiners report
Homogeneous catalysis was usually identified in part (a). In (b), few got the correct mechanism for \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\), namely the fact that there is a change in the oxidation state. The command term outline needs to be differentiated from state. Correct disadvantages were usually identified and for Fe(s) most knew that the mechanism involved reactants being adsorbed onto the surface. Outline is an objective 2 command term, as given on P.11 of the guide and equates to giving a brief account or summary. Hence simply stating heterogeneous for the mechanism was incorrect for Fe(s) for example. Candidates need to pay close attention to the command terms as part of their examination preparation.
Homogeneous catalysis was usually identified in part (a). In (b), few got the correct mechanism for \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\), namely the fact that there is a change in the oxidation state. The command term outline needs to be differentiated from state. Correct disadvantages were usually identified and for Fe(s) most knew that the mechanism involved reactants being adsorbed onto the surface. Outline is an objective 2 command term, as given on P.11 of the guide and equates to giving a brief account or summary. Hence simply stating heterogeneous for the mechanism was incorrect for Fe(s) for example. Candidates need to pay close attention to the command terms as part of their examination preparation.

