| Date | May 2018 | Marks available | 1 | Reference code | 18M.3.sl.TZ2.14 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Deduce | Question number | 14 | Adapted from | N/A |
Question
One method of producing biodiesel is by a transesterification process.
Deduce the equation for the transesterification reaction of pentyl octanoate, C7H15COOC5H11, with methanol.
Outline why the ester product of this reaction is a better diesel fuel than pentyl octanoate.
Markscheme
C7H15COOC5H11(l) + CH3OH(l) → C7H15COOCH3(l) + C5H11OH(l)
OR
C13H26O2(l) + CH4O(l) → C9H18O2(l) + C5H12O(l)
OR
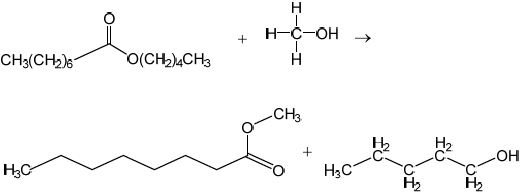
Accept correct equation in any format eg, skeletal, condensed structural formula, etc.
Accept equations with equilibrium arrow.
[1 mark]
less viscous «and so does not need to be heated to flow»
OR
less likely to undergo incomplete combustion
OR
fewer intermolecular/London/dispersion forces
OR
vaporizes easier
Ignore equation and products in 14a.
Accept “van der Waals’/vdW” for “London”.
[1 mark]

