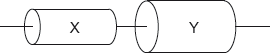| Date | May 2021 | Marks available | 2 | Reference code | 21M.2.SL.TZ1.3 |
| Level | Standard level | Paper | Paper 2 | Time zone | 1 |
| Command term | Calculate | Question number | 3 | Adapted from | N/A |
Question
A mass of 1.0 kg of water is brought to its boiling point of 100 °C using an electric heater of power 1.6 kW.
A mass of 0.86 kg of water remains after it has boiled for 200 s.
The electric heater has two identical resistors connected in parallel.
The circuit transfers 1.6 kW when switch A only is closed. The external voltage is 220 V.
The molar mass of water is 18 g mol−1. Estimate the average speed of the water molecules in the vapor produced. Assume the vapor behaves as an ideal gas.
State one assumption of the kinetic model of an ideal gas.
Estimate the specific latent heat of vaporization of water. State an appropriate unit for your answer.
Explain why the temperature of water remains at 100 °C during this time.
The heater is removed and a mass of 0.30 kg of pasta at −10 °C is added to the boiling water.
Determine the equilibrium temperature of the pasta and water after the pasta is added. Other heat transfers are negligible.
Specific heat capacity of pasta = 1.8 kJ kg−1 K−1
Specific heat capacity of water = 4.2 kJ kg−1 K−1
Show that each resistor has a resistance of about 30 Ω.
Calculate the power transferred by the heater when both switches are closed.
Markscheme
Ek = « » = «J» ✓
v = «» = 720 «m s−1» ✓
particles can be considered points «without dimensions» ✓
no intermolecular forces/no forces between particles «except during collisions»✓
the volume of a particle is negligible compared to volume of gas ✓
collisions between particles are elastic ✓
time between particle collisions are greater than time of collision ✓
no intermolecular PE/no PE between particles ✓
Accept reference to atoms/molecules for “particle”
«mL = P t» so «» = 2.3 x 106 «J kg-1» ✓
J kg−1 ✓
«all» of the energy added is used to increase the «intermolecular» potential energy of the particles/break «intermolecular» bonds/OWTTE ✓
Accept reference to atoms/molecules for “particle”
use of mcΔT ✓
0.86 × 4200 × (100 – T) = 0.3 × 1800 × (T +10) ✓
Teq = 85.69«°C» ≅ 86«°C» ✓
Accept Teq in Kelvin (359 K).
«Ω» ✓
Must see either the substituted values OR a value for R to at least three s.f.
use of parallel resistors addition so Req = 15 «Ω» ✓
P = 3200 «W» ✓