| Date | May 2018 | Marks available | 1 | Reference code | 18M.1.SL.TZ2.13 |
| Level | Standard level | Paper | Paper 1 | Time zone | 2 |
| Command term | Question number | 13 | Adapted from | N/A |
Question
A sealed container contains water at 5 °C and ice at 0 °C. This system is thermally isolated from its surroundings. What happens to the total internal energy of the system?
A. It remains the same.
B. It decreases.
C. It increases until the ice melts and then remains the same.
D. It increases.
Markscheme
A
Examiners report
Syllabus sections
- 17N.1.HL.TZ0.9: The fraction of the internal energy that is due to molecular vibration varies in the...
-
17N.2.SL.TZ0.4b.ii:
Outline the difference between the molecular structure of a solid and a liquid.
- 22M.1.HL.TZ2.11: Water at room temperature is placed in a freezer. The specific heat capacity of water is...
- 17N.3.SL.TZ0.1b.i: Determine the gradient of the line at a temperature of 80 °C.
- 18M.1.SL.TZ2.12: A container that contains a fixed mass of an ideal gas is at rest on a truck. The truck...
- 17N.3.SL.TZ0.1c.i: Calculate the energy required to raise the temperature of the water from 75 °C to 85 °C.
-
17N.3.SL.TZ0.1c.ii:
Using an appropriate error calculation, justify the number of significant figures that should be used for your answer to (c)(i).
-
22M.2.SL.TZ1.2a:
Estimate the power input to the heating element. State an appropriate unit for your answer.
- 17M.1.SL.TZ1.10: A liquid is initially at its freezing point. Energy is removed at a uniform rate from the...
- 17M.1.SL.TZ2.10: The graph shows the variation with time t of the temperature T of two samples, X and Y. X and...
- 22M.2.SL.TZ2.2b.ii: Discuss, for this process, the changes that occur in the internal energy of the gas.
- 22M.1.SL.TZ1.10: A driver uses the brakes on a car to descend a hill at constant speed. What is correct...
-
22M.1.SL.TZ1.11:
Two blocks, X and Y, are placed in contact with each other. Data for the blocks are provided.
X has a mass . What is the mass of Y?
A.
B.
C.
D.
- 17N.1.SL.TZ0.10: A 1.0 kW heater supplies energy to a liquid of mass 0.50 kg. The temperature of the liquid...
- 17M.1.SL.TZ1.15: Two pulses are travelling towards each other. What is a possible pulse shape when the...
- 16N.2.SL.TZ0.3a: Define internal energy.
- 18N.1.SL.TZ0.10: A 700 W electric heater is used to heat 1 kg of water without energy losses. The specific...
- 18N.1.HL.TZ0.8: A solid substance has just reached its melting point. Thermal energy is supplied to the...
-
18N.2.SL.TZ0.7a:
Distinguish between the internal energy of the oxygen at the boiling point when it is in its liquid phase and when it is in its gas phase.
- 18N.2.SL.TZ0.7b.i: Calculate, in kW, the heater power required.
- 18N.2.HL.TZ0.9b.i: Calculate, in kW, the heater power required.
- 22M.1.SL.TZ2.13: System X is at a temperature of 40 °C. Thermal energy is provided to system X until it...
- 19M.2.HL.TZ2.4dii: Suggest, in terms of conservation of energy, the cause for the above change.
- 19M.1.SL.TZ1.12: Boiling water is heated in a 2 kW electric kettle. The initial mass of water is 0.4 kg....
- 19M.1.SL.TZ2.10: A substance changes from the solid phase to the gas phase without becoming a liquid and...
-
18M.2.SL.TZ1.2b.i:
Calculate, in kg, the mass of the gas.
-
18M.1.SL.TZ1.11:
What are the units of the ratio ?
A. no units
B. k
C. k–1
D. k–2
- 16N.1.SL.TZ0.10: Energy is supplied at a constant rate to a fixed mass of a material. The material begins as a...
-
21N.1.SL.TZ0.11:
A mass of a liquid of specific heat capacity flows every second through a heater of power . What is the difference in temperature between the liquid entering and leaving the heater?
A.B.
C.
D.
-
19M.1.HL.TZ2.12:
A liquid of mass m and specific heat capacity c cools. The rate of change of the temperature of the liquid is k. What is the rate at which thermal energy is transferred from the liquid?
A.
B.
C.
D. kmc
-
17N.3.SL.TZ0.1b.ii:
State the unit for the quantity represented by the gradient in your answer to (b)(i).
-
17M.2.SL.TZ1.1a.ii:
Some of the gravitational potential energy transferred into internal energy of the skis, slightly increasing their temperature. Distinguish between internal energy and temperature.
-
18M.1.SL.TZ2.11:
The graph shows how the temperature of a liquid varies with time when energy is supplied to the liquid at a constant rate P. The gradient of the graph is K and the liquid has a specific heat capacity c.
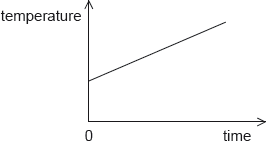
What is the mass of the liquid?
A.
B.
C.
D.
-
18N.2.HL.TZ0.9a:
Distinguish between the internal energy of the oxygen at the boiling point when it is in its liquid phase and when it is in its gas phase.
-
17M.2.HL.TZ2.3c.i:
The mass of the resistance wire is 0.61 g and its observed temperature rise is 28 K. Estimate the specific heat capacity of the wire. Include an appropriate unit for your answer.
-
22M.2.SL.TZ1.2b:
Outline whether your answer to (a) is likely to overestimate or underestimate the power input.
-
20N.1.SL.TZ0.13:
A bicycle of mass comes to rest from speed using the back brake. The brake has a specific heat capacity of and a mass . Half of the kinetic energy is absorbed by the brake.
What is the change in temperature of the brake?
A.
B.
C.
D.
-
19N.1.SL.TZ0.9:
A mass of water is at a temperature of 290 K. The specific heat capacity of water is . Ice, at its melting point, is added to the water to reduce the water temperature to the freezing point. The specific latent heat of fusion for ice is . What is the minimum mass of ice that is required?
A.
B.
C.
D.
-
20N.2.SL.TZ0.3c:
Calculate the mass of the oil that remains unfrozen after minutes.
-
21M.2.SL.TZ1.3b.i:
Estimate the specific latent heat of vaporization of water. State an appropriate unit for your answer.
- 21M.2.SL.TZ1.3b.ii: Explain why the temperature of water remains at 100 °C during this time.
-
21M.2.SL.TZ1.3c:
The heater is removed and a mass of 0.30 kg of pasta at −10 °C is added to the boiling water.
Determine the equilibrium temperature of the pasta and water after the pasta is added. Other heat transfers are negligible.
Specific heat capacity of pasta = 1.8 kJ kg−1 K−1
Specific heat capacity of water = 4.2 kJ kg−1 K−1 -
20N.2.SL.TZ0.3a(i):
Calculate the thermal energy transferred from the sample during the first minutes.
- 20N.2.SL.TZ0.3b: The sample begins to freeze during the thermal energy transfer. Explain, in terms of the...
-
20N.2.SL.TZ0.3a(ii):
Estimate the specific heat capacity of the oil in its liquid phase. State an appropriate unit for your answer.
-
21M.1.SL.TZ2.12:
A piece of metal at a temperature of is dropped into an equal mass of water at a temperature of in a container of negligible mass. The specific heat capacity of water is four times that of the metal. What is the final temperature of the mixture?
A.
B.
C.
D.
-
21M.1.SL.TZ1.11:
When 40 kJ of energy is transferred to a quantity of a liquid substance, its temperature increases by 20 K. When 600 kJ of energy is transferred to the same quantity of the liquid at its boiling temperature, it vaporizes completely at constant temperature. What is
for this substance?
A. 15 K−1
B. 15 K
C. 300 K−1
D. 300 K
-
17N.2.SL.TZ0.4b.i:
Determine the energy required to melt all of the ice from –20 °C to water at a temperature of 0 °C.
Specific latent heat of fusion of ice = 330 kJ kg–1
Specific heat capacity of ice = 2.1 kJ kg–1 k–1
Density of ice = 920 kg m–3 - 19M.1.SL.TZ1.10: Energy is transferred to water in a flask at a rate P. The water reaches boiling point and...
-
19M.1.SL.TZ1.11:
An insulated tube is filled with a large number n of lead spheres, each of mass m. The tube is inverted s times so that the spheres completely fall through an average distance L each time. The temperature of the spheres is measured before and after the inversions and the resultant change in temperature is ΔT.
What is the specific heat capacity of lead?
A.
B.
C.
D.
-
19M.2.SL.TZ1.4b.ii:
Determine the atmospheric pressure. Give a suitable unit for your answer.
-
17M.2.HL.TZ1.6d:
At the instant of impact the meteorite which is made of ice has a temperature of 0 °C. Assume that all the kinetic energy at impact gets transferred into internal energy in the meteorite. Calculate the percentage of the meteorite’s mass that melts. The specific latent heat of fusion of ice is 3.3 × 105 J kg–1.
- 17M.1.SL.TZ2.11: A mass m of ice at a temperature of –5 °C is changed into water at a temperature of 50...
- 17M.2.HL.TZ2.3c.ii: Suggest one other energy loss in the experiment and the effect it will have on the value for...
-
21N.1.HL.TZ0.9:
An insulated container of negligible mass contains a mass 2M of a liquid. A piece of a metal of mass M is dropped into the liquid. The temperature of the liquid increases by 10 °C and the temperature of the metal decreases by 80 °C in the same time.
What is ?
A. 2B. 4
C. 8
D. 16
- 21N.1.SL.TZ0.10: A liquid is vaporized to a gas at a constant temperature. Three quantities of the substance...
- 21N.2.SL.TZ0.2a: State what is meant by the internal energy of an ideal gas.
-
21N.2.HL.TZ0.4c.i:
Estimate the power, in kW, that is available from the plutonium at launch.
-
21N.2.HL.TZ0.5d.ii:
The mass of the wire is 18 g. The specific heat capacity of copper is 385 J kg−1 K−1. Estimate the increase in temperature of the wire.
-
21N.2.HL.TZ0.6d.i:
Show that the mass of a nitrogen molecule is 4.7 × 10−26 kg.
-
22M.2.SL.TZ1.2c:
Discuss, with reference to the molecules in the liquid, the difference between milk at 11 °C and milk at 84 °C.

